Full Length Research Article
Antidiabetic Activity and Pancreatic Histopathology Analysis of Averrhoa bilimbi L. Leaves Extract on Alloxan-Induced Diabetes on Wistar Mice
Tridiganita Intan Solikhah1,2*, Ragil Kusnandar Miftakhurrozaq3, Aswin Rafif Khairullah4
Adv. life sci., vol. 11, no. 3, pp. 669-673, August 2024
*- Corresponding Author: Tridiganita Intan Solikhah (tridiganita-intan-s@fkh.unair.ac.id)
Authors' Affiliations
2. School of Health and Life Science, Universitas Airlangga – Indonesia
3. Faculty of Medicine, Universitas Jember – Indonesia
4. Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor – Indonesia
[Date Received: 25/01/2024; Date Revised: 21/06/2024; Date Published: 10/07/2024]
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Diabetes mellitus is a multi-etiological metabolic disorder characterized by chronic hyperglycemia, total or relative insulin deficiency, and further complications by metabolic disorders. Drug therapy for diabetes mellitus is costly and fraught with potential side effects. The aim of this research is to investigate antidiabetic and histopathological analysis of ethanolic A. bilimbi leaves extract in alloxan induced diabetic mice.
Methods: Wuluh starfruit leaves is one of Indonesia's plants that can be used to treat diabetes mellitus. Thirty male Wistar mice were divided into five groups as follows: Negative control group that didn't receive any treatment, Diabetes control group that received alloxan 150 mg/kg, Positive control group that received glibenclamide 600 μg/kg, and lastly the two test groups that received A. bilimbi leaves extract 150 and 300 mg/kg orally for 14 days. After extract administration, blood glucose and histopathological alterations in the pancreas of diabetic mice were observed.
Results: Alloxan to the diabetes control group significantly raised blood glucose levels compared to the negative control group. Administration of wuluh starfruit leaves extract and glibenclamide could significantly lower blood sugar levels compared to the diabetic control group. Administration of wuluh starfruit leaves extract treatment and glibenclamide also showed favorable effect on the histopathological changes of the pancreas in alloxan induced diabetes.
Conclusion: The study concludes the administration of wuluh starfruit leaves extract (150 and 300 mg/kg) and glibenclamide could reduce blood glucose levels and can help with cell regeneration and protecting pancreatic cells from damage caused by alloxan.
Keywords: A. bilimbi leaves extract; Alloxan; Diabetes; Pancreatic histopathology
Diabetes mellitus or hyperglycemic state is a multi-etiological metabolic disorder characterized by chronic hyperglycemia, total or relative insulin deficiency, and further complications from metabolic disorders. Diabetes mellitus is one of the main causes of death in developed and developing countries nowadays [1, 2]. According to a study by the International Diabetes Federation (IDF), high blood pressure and tobacco use are the top two risk factors for early death globally. Elevated blood glucose is in third place [3, 4].
Currently, in clinical practice, insulin and oral chemical antidiabetic agents (a-glucosidase inhibitors, sulfonylureas, insulin sensitizers, biguanides, etc.) are used as a treatment for diabetes mellitus. Many of them have some intolerable disadvantages and adverse reactions, such as liver failure, kidney failure, hypoglycemia, diarrhea, and lactic acidosis. Therefore, the research for new compounds with better efficiency has gained more attention as new therapeutic antidiabetic drugs for diabetes mellitus patients to protect patients from the adverse effects of these synthetic agents [5].
Indonesia owns enormous natural resources [6- 8]. Wuluh starfruit (Averrhoa bilimbi L.) is one of the plants in Indonesia that can be used to treat diabetes mellitus [9]. Almost all parts of the wuluh starfruit plant can be utilized, one of which is the leaves. Wuluh starfruit leaves are one of the popular medicinal plants in traditional medicine in Malaysia, Argentina, Australia, Brazil, India, Philippines, Singapore, Thailand, and Venezuela and have been used for centuries to treat various diseases [10]. Wuluh starfruit entered Indonesia and grows abundantly throughout Indonesia, one of which is Bali. Wuluh starfruit leaves contain flavonoids, saponins, tannins, sulfur, formic acid, peroxidase, calcium oxalate and potassium citrate. Wuluh starfruit leaves can be used as a medication for rheumatism, stroke, and cough, and act as anti-inflammatory, analgesic, anti-hypertension, anti-diabetes, and anti-hyperlipidemia agents as well [11].
Several studies have demonstrated that wuluh starfruit (Averrhoa bilimbi L.) leaves have the potential as an antidiabetic drug. A study by Alipin et al., [12] showed that a dosage of the combination of Averrhoa bilimbi fruit extract and Curcuma xanthorrhiza Rhizome (767.5 mg/kg bw) significantly reduced blood glucose levels (54.86 ± 34.61) and improved the histopathology of the pancreas, as evidenced by an increase in the number Langerhans (9.33 ± 0.58) and diameter (53.34 ± 8.82) of Langerhans islets and a decrease in the degree of insulitis (p<0.05). The results of a study conducted by Sutrisna and Sujono [13] also demonstrated that the combination of ethanol extract from wuluh starfruit leaves and Tapak Dara leaves obtained using the cold maceration method with doses of 40:80 and 80:40 mg/200 g BW could reduce blood glucose levels in 7 days in diabetic mice injected with alloxan.
Based on those studies above, in this study, further research will be conducted on testing the antidiabetic activity of the ethanol extract of wuluh starfruit (Averrhoa bilimbi L.) leaves which was obtained by the maceration method using mice as different experimental animals from previous studies, with different doses variations of the extract.
Tools and materials
The tools and materials used in this study were wuluh starfruit (A. bilimbi) leaves from Unggahan Village, Banjaragung Hamlet, Puri District, Mojokerto Regency. Sampling of wuluh starfruit leaves was performed purposively without comparing them with the same plants from other areas. Other materials were 0.9% NaCl (PT Otsuka Indonesia), ethanol 96% (CV. Setya Jaya Abadi), Whatman filter paper no. 1 (CV. Unichem Arkatama), CMC Na (CV. Duta Perkasa Mandiri), distilled water (CV Zrafindo Sejahtera), alloxan (PT Nitra Kimia), digital scale, 1 cc syringe (OneMed), glucometer (Accu Chek Instant), glucose test strip, gloves (OneMed), mask (OneMed), mouse cage, feed container, drinker, and feeding tube.
Experimental animals
White, healthy male Wistar mice, aged ± 3 months, have never been used for other experiments and weighing between 25-35 grams were used in this study. Before being given treatment, the Wistar mice were adapted to the environment for 7 days. Feed was given at 4 g/mice/day and drinking water were provided ad libitum. Universitas Airlangga Faculty of Dental Medicine Health Research Ethical Clearance Commision approved all procedures of this study (835/HRECC.FODM/VII/2023)
Preparation of plant extracts
The extraction procedure follows the procedure from Solikhah et al., [14], to create dry powder, A. bilimbi leaves were cleaned, cooked at 60°C, then crushed. The powder from Wuluh starfruit leaves was soaked in ethanol 96% at a ratio of 1:7, agitated multiple times, and kept at room temperature for 24 hours. It was filtered and the macerate was separated after 24 hours. Ethanol 96% was added to the dregs during processing and re-maceration in a ratio of 1:4. After filtering, the entire macerate was evaporated at 60 °C to produce a thick extract. To protect the thick extract from deterioration, it was put in a beaker glass container wrapped with aluminum foil and stored in the freezer. To create the extract, CMC Na 0.5% was employed as the solvent. The yield percentage of the extract was calculated using the formula below:
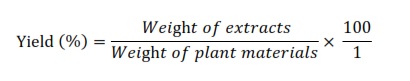
Antidiabetic test
Diabetic Wistar mice were formed after 7 days post-adaptation. Adapted Wistar mice were injected with alloxan intraperitoneally at a dose of 150 mg/kg BW which had been dissolved in 0.9% NaCl solution, except for the K0 group which was not induced by alloxan. After the 5th day post-induction, Wistar mice with fasting blood glucose levels ≥ 200 mg/dL were considered diabetic and included in the study, then divided into five groups with 6 Wistar mice per group [15] as follows: Negative control group (treatment of mice without administration of alloxan), diabetes control group (treatment of Wistar mice with alloxan 150 mg/kg BW), positive control group (Administration of glibenclamide 600 μg/kg BW), treatment control group (Administration of 150 and 300 mg/kg BW A. bilimbi leaves extract).
The mice's blood glucose levels were measured on days 1, 7 and 14 after drug and extract administration. Blood samples were collected, and blood glucose was measured with a glucose autoanalyzer kit.
Histopathological examination of the pancreas
After administering the extract and glibenclamide for 14 days, all Wistar mice groups given light anesthesia. Next, dislocate the neck and take the pancreas organ. The pancreas was washed, fixed, dehydrated, and washed multiple times with alcohol 90%, absolute ethanol 95% and xylol. Furthermore, the infiltration process was performed by adding paraffin 3 times, then followed by embedding. Next, sections were conducted using a microtome with a thickness of 5 mm, then proceeded with the initialization stage. Slides were then stained with hematoxylin and eosin (HE). The stained sections were qualitatively evaluated using a photo microscope and digital camera [17].
The findings demonstrated that giving alloxan to the diabetes control group significantly raised blood glucose levels on days 7, and 14 compared to the negative control group (P < 0.05). Meanwhile, administration of glibenclamide and wuluh starfruit leaf extract could significantly lower blood sugar levels compared to the diabetic control group on days 7 and 14 (P < 0.05). At the end of experiment (14th day) blood glucose level was (112,6 ± 8,02) mg/dL, (161,2 ± 10,08) and (191,6 ± 7,1) mg/dL of the groups treated with the doses of glibenclamide, A. bilimbi leaves extract 300 and 150 mg/kg respectively (Table 1).
A dose of A. bilimbi leaves extract 150 mg/kg BW was given to a group, abnormal cell morphologies were seen, the borders of the islets of Langerhans were not clear, the number of cells began to decrease, and necrosis occurred in most cells. At a dose of A. bilimbi leaves extract 300 mg/kg BW, it appeared that the shape of the cells looked normal and the border of the islets of Langerhans became clearer. In addition, the number of cells began to increase, and no necrosis occurred in the cells. In the positive control glibenclamide, normal cell shapes were observed. There were changes in the organ border of the islets of Langerhans began to be seen clearly, and necrosis began to decrease. This showed a decrease in the level of damage compared to wuluh starfruit leaves extract with doses of 150 and 300 mg/kg BW (Figure 1).
Figures & Tables
Type 1 diabetes was induced using alloxan after 5 days post-induction and the Wistar mice's blood glucose levels were measured using a glucose test strip. Mice with a fasting blood sugar level of 200 mg/dL were considered diabetic and were included in this study. A. bilimbi L. extract was administered orally to diabetic mice for 14 days without any observed mortality.
One of the many substances frequently injected into laboratory animals to induce diabetes is alloxan [17]. With an intraperitoneal injection of alloxan at a dose of 150 mg/kg BW, 80% of animal subjects were successfully given diabetes, and only 10% of them died as a result [18, 19]. Alloxan causes a hyperglycemic response in as little as two to three days [9, 20]. Alloxan may have an impact on how much and how well the pancreatic beta cells produce insulin [18]. Once alloxan has successfully entered the pancreatic beta cells, it causes reactive oxygen species to be produced and glucokinase to be inhibited, which leads to disastrous outcomes. Depolarization is a step in the process that allows more calcium to enter pancreatic beta cells through voltage-dependent calcium channels. High levels of insulin release were found to be considerably influenced by high intracellular Ca2+ levels [17, 18]. From the first to the fourteenth day, the dosages of glibenclamide (600 μg/kg BW) and A. bilimbi leaf extract (150 and 300 mg/kg BW) were given. Blood glucose levels dropped in all groups. Glibenclamide was most efficient in lowering blood glucose levels, followed by A. bilimbi leaf extract at doses of 300 and 150 mg/kg BW. One sulfonylurea medication used to treat diabetes is glibenclamide [9, 21, 22]. In individuals with hyperglycemia, this medication increases the pancreatic beta cells’ ability to produce insulin [23]. Sulfonylurea receptor-1 (SUR1), a regulatory subunit of ATP-sensitive potassium (KATP) channels found in pancreatic beta cells, is the receptor that glibenclamide binds to. Calcium channels will open as the cell membrane depolarizes. It increases the beta cells’ intracellular calcium content and enhances the release of insulin [24, 25].
The effect of reducing blood sugar levels is due to the presence of bioactive compounds contained in the ethanol extract of wuluh starfruit leaves (A. bilimbi L.) such as alkaloids, flavonoids, tannins, polyphenols, and quinones. It has been demonstrated that alkaloids can regenerate injured pancreatic cells. With changes in pancreatic tissue, the body will produce more insulin, which will allow blood glucose to enter the cells and lower blood sugar levels [26]. The ethanol leaves of wuluh starfruit (A. bilimbi L.) contain flavonoids that may function as antioxidants. Since flavonoids are known to have antioxidant action, it is thought that they can shield the body from the harm that reactive oxygen species produce, hence reducing degenerative illnesses like diabetes mellitus. In order to reverse insulin insufficiency, flavonoids are thought to be crucial in boosting the activity of antioxidant enzymes and regenerating damaged pancreatic cells [9, 14, 26]. A. bilimbi leaves have flavonoids that may enhance insulin receptor sensitivity. Therefore, flavonoids have a positive impact on the condition of diabetes mellitus [27]. By devoting a hydrogen atom from the aromatic hydroxyl group (-OH) of polyphenols, antioxidants in them may lessen oxidative stress by blocking the chain of superoxide conversion into hydrogen superoxide. This allows free radicals to be bound and eliminated from the body through the excretory system. On the other hand, tannins are known to increase the metabolism of fat and glucose [28], preventing the buildup of these two calorie-rich substances in the blood. Additionally, tannins have an astringent or chelating effect, which can shorten the epithelial membrane of the small intestine and decrease food essence absorption [29]. This inhibits the absorption of glucose and slows the rate at which blood sugar levels rise. Wuluh starfruit contains active polyphenols with antioxidant and hypoglycemic properties. Free radicals are snatched up by polyphenolic substances, which also lessen oxidative stress [30]. By lowering oxidative stress and ROS, phytochemical substances may act through a variety of methods to reduce the problems associated with diabetes [31].
Wuluh starfruit leaf extract is a potential alternative treatment for diabetes. Administration of wuluh starfruit leaves extract (150 and 300 mg/kg) and glibenclamide could reduce blood glucose levels in mice with alloxan-induced diabetes. A. bilimbi leaf extract may help with cell regeneration and protecting pancreatic cells from damage caused by alloxan. To fully comprehend the impact of wuluh starfruit leaf extract on diabetic patients, additional research is required to examine its antioxidant activity in conjunction with the expression of certain genes or biomarkers connected to diabetes.
Acknowledgement
This research was supported through internal research funding from Universitas Airlangga (Grant number 254/UN3/2023).
Author Contributions
All Authors were responsible for the study design, data gathering, data analysis, manuscript preparation, and editing of the manuscript.
The author declare that there is no conflict of interest regarding the publication of this paper.
- Luo Z, Fu C, Li T, Gao Q, Miao D, et al. Hypoglycemic effects of licochalcone A on the streptozotocin-induced diabetic mice and its mechanism study. Journal of Agricultural and Food Chemistry, (2021); 69(8): 2444–2456.
- Lotfy M, Adeghate J, Kalasz H, Singh J, Adeghate E. Chronic complications of diabetes mellitus: a mini review. Current Diabetes Reviews, (2017); 13(1): 3–10.
- IDF (International Diabetes Federation) Diabetes Atlas-7th Edition, (2015).
- Tafesse TB, Hymete A, Mekonnen Y, Tadesse M. Antidiabetic activity and phytochemical screening of extracts of the leaves of ajuga remota benth on alloxan-induced diabetic mice. BMC Complementary Medicine and Therapies, (2017); 17(1): 1–9.
- Zhang Y, Wu L, Ma Z, Cheng J, Liu J. Anti-hyperlipidemic activities of flavonoids from corn silk on STZ-Induced diabetic mice. Molecules, (2016); 21(1): 7–10.
- Khairullah AR, Solikhah TI, Ansori ANM, Hanisia RH, Puspitarani GA, et al. 2021. Medicinal importance of Kaempferia galanga L. (Zingiberaceae): A comprehensive review. Journal of Herbmed Pharmacology, (2021); 10(3): 281–288.
- Khairullah AR, Solikhah TI, Ansori ANM, Fadholly A, Ramandinianto SC, et al. A review of an important medicinal plant : Alpinia galanga (L.) willd. Systematic Reviews in Pharmacy, (2020); 11(10): 387–395.
- Safira A, Widayani P, An-najaaty D, Rani CAM, Septiani M, et al. A review of an important plants: Annona squamosa leaf. Pharmacognosy Journal, (2022); 14(2): 456–463.
- Solikhah TI, Setiawan B, Ismukada DR. Antidiabetic activity of papaya leaf extract (Carica Papaya L.) isolated with maceration method in alloxan-induces diabetic mice. Systematic Reviews in Pharmacy, (2020); 11(9): 774–778.
- Garg M, Chaudhary SK, Kumari S, Goyal A. Phytochemical, biological and traditional claims on Averrhoa bilimbi: An overview. Indian Journal of Pharmaceutical Sciences, (2022); 84(3): 532-536.
- Roy A, Geetha RV, Lakshmi T. Averrhoa bilimbi Linn-nature’s drug store-A pharmacological review. International Journal of Drug Development and Research, (2011); 3(3): 101–106.
- Alipin K, Istiqamah N, Maryani A, Madihah. The potential of combined Curcuma xanthorrhiza rhizome and Averrhoa bilimbi fruit extract on decreasing blood glucose levels, insulitis degree and liver structure repair of diabetic male wistar rats streptozotocin induced. Journal of Diabetes & Metabolism, (2019); 10(10): 1–7.
- Sutrisna EM, Sujono TA. The combination of belimbing wuluh fruit (Averrhoa bilimbi L.) and leaves of tapak dara (Catharanthus roseus G.) from Indonesia as a candidate hypoglycemic agents and thin layer chromatography profiles. Biomedical and Pharmacology Journal, (2015); 8(1): 39–46.
- Solikhah TI, Wijaya TA, Pavita DA, Kusnandar R, Wijaya A, et al. The effect of sapodilla leaf extract (Manilkara zapota L.) on lipid profiles of alloxan-induced diabetic mice. Pharmacognosy Journal, (2023); 15(2): 286–289.
- Balamurugan K, Nishanthini A, Mohan VR. Antidiabetic and antihyperlipidaemic activity of ethanol extract of Melastoma malabathricum Linn. leaf in alloxan induced diabetic rats. Asian Pacific Journal of Tropical Biomedicine, (2014); 4(Suppl 1): S442–S448.
- Jaiswal YS, Tatke PA, Gabhe SY, Vaidya AB. Antidiabetic activity of extracts of Anacardium occidentale Linn. leaves on n-streptozotocin diabetic rats. Journal of Traditional and Complementary Medicine, (2017); 7(4): 421–427.
- Ighodaro OM, Adeosun AM, Akinloye OA. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina (Buenos Aires), (2017); 53(6): 365–374.
- Radenković M, Stojanović M, Prostran M. Experimental diabetes induced by alloxan and streptozotocin: The current state of the art. Journal of Pharmacological and Toxicological Methods, (2016); 78(1): 13–31.
- Solikhah TI, Rani CA, Septiani M, Putra YA, Rachmah Q, et al. Antidiabetic of Hylocereus polyrhizus peel ethanolic extract on alloxan induced diabetic mice. Iraqi Journal of Veterinary Sciences, (2022); 36(3): 797–802.
- Dey P, Saha MR, Chowdhuri SR, Sen A, Sarkar MP, et al. Assessment of anti-diabetic activity of an ethnopharmacological plant Nerium oleander through alloxan induced diabetes in mice. Journal of Ethnopharmacology, (2015); 161(1): 128–137.
- Sola D, Rossi L, Schianca GPC, Maffioli P, Bigliocca M, et al. 2015. Sulfonylureas and their use in clinical practice. Archives of Medical Science, (2015); 11(4): 840–848.
- Tawfeek HM, Roberts M, Hamd MA, El Abdellatif AA, Younis MA. 2018. Glibenclamide mini-tablets with an enhanced pharmacokinetic and pharmacodynamic performance. AAPS PharmSciTech, (2018); 19(7): 2948–2960.
- Zhou J, Kang X, Luo Y, Yuan Y, Wu Y, et al. Glibenclamide-induced autophagy inhibits its insulin secretion-improving function in β cells. International Journal of Endocrinology, (2019); 2019(1): 1–8.
- Liu H, Wang S, Wu Z, Huang Z, Chen WY, et al. Glibenclamide, a diabetic drug, prevents acute radiation induced liver injury of mice via up-regulating intracellular ROS and subsequently activating Akt-NF-κB pathway. Oncotarget, (2017); 8(25): 40568–40582.
- Sreejesh PG, Thampi BH, Sreekumaran E. 2017. Hypoglycaemic effect of glibenclamide: A critical study on the basis of creatinine and lipid peroxidation status of streptozotocin-induced diabetic rat. Indian Journal of Pharmaceutical Sciences, (2017); 79(5): 768–777.
- Solikhah TI, Solikhah GP. 2021. Effect of Muntingia calabura L. leaf extract on blood glucose levels and body weight of alloxan-induced diabetic mice. Pharmacognosy Journal, (2021); 13(6): 1450–1455.
- Varshney R, Mishra R, Das N, Sircar D, Roy P. A comparative analysis of various flavonoids in the regulation of obesity and diabetes: An in vitro and in vivo study. Journal of Functional Foods, (2019); 59(1): 194–205.
- Li Y, Zhu L, Guo C, Xue M, Xia F, et al. Dietary intake of hydrolyzable tannins and condensed tannins to regulate lipid metabolism. Mini-Reviews in Medicinal Chemistry, (2022); 22(13): 1789–1802.
- Tandi J, Danthy R, Kuncoro H. Effect of ethanol extract from purple eggplant skin (Solanum melongena L) on blood glucose levels and pancreatic? cells regeneration on white rats male hypercholesterolemia-diabetic. Research Journal of Pharmacy and Technology, (2019); 12(6): 2936–2942.
- Rivas F, Poblete-Aro C, Pando ME, Allel MJ, Fernandez V, et al. Effects of polyphenols in aging and neurodegeneration associated with oxidative stress. Current Medicinal Chemistry, (2022); 29(6): 1045–1060.
- Forni C, Facchiano F, Bartoli M, Pieretti S, Facchiano A, et al. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. BioMed Research International, (2019); 2019(1): 8748253.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0![]()





