Research Article
In-vitro Production of Cabbage and Cauliflower
Zahida Qamar1*, Idrees Ahmad Nasir1, Ghulam Zahra Jahangir2, Tayyab Husnain1
Adv. life sci., vol. 1, no. 2, pp. 112-118, February 2014
*-Corresponding Author: Zahida Qamar (Email: florilab5@gmail.com)
Author Affiliations
2- Centre for Applied Molecular Biology, 87-West Canal Bank Thokar Niazbaig, Lahore-53700 – Pakistan
[Date Received: 21/01/2014; Date Revised:13/02/2014; Date Published Online: 02/25/2014]
Abstract
Introduction
Methods
Results
Discussion
References
Abstract
Background: An efficient method of artificial mass propagation was optimized for two very nutritious vegetables of Pakistan, the cabbage and cauliflower. Being an agrarian economy, Pakistan’s more than half of the population depends directly or indirectly on agricultural products.
Methodology: Hypocotyls of germinating of seeds (5-7 days old seedlings) were used as explants. Murashige and Skoog basal medium was supplemented with different concentrations of auxin (2,4 Dichlorophenoxy acetic acid) and cytokinin (benzyl amino purine) in combination to study the callus forming tendency of cabbage and cauliflower; and found better if used in ratio of 2:1 (at least) respectively.
Results: For evaluation of regeneration potential Kinetin, Zeatin, Gibrellic acid-3, and Indole acetic acid were found good in combination with benzyl amino purine. Higher (than auxin) concentration of cytokinin was found essential to obtain good regeneration response of callus.
Conclusion: Effectively concluded that these techniques can be used to raise the disease free stock of cabbage and cauliflower for genetic improvement of in-vitro bulk of varieties on commercial scale in a very brief time span.
Keywords: Cabbage, Cauliflower, Mass production, Murashige and Skoog basal medium
Introduction
Cabbage and cauliflower are two of several vegetables in the species Brassica oleracea of family Brassicaceae [1]. These are grown, in Pakistan, for their edible value and are important cash crops of Pakistan [2]. These are usually grown as a winter crops and sometimes also as summer vegetables. Being an agrarian economy, Pakistan’s more than half of the population depends directly or indirectly on agricultural products. Vegetable production carries a valuable status in edible crops of Pakistan. Although the cabbage and cauliflower are among minor crops and are mostly grown in small farms even then Cauliflower is one of the most cultivated vegetable in the Punjab. Prevalently landless and poor farmers prefer to grow vegetables on small farms because vegetable production gives higher returns than cereals and other crops. High labor input is an important concern of farming vegetables. As landless labor class pervades the rural areas therefore these crops can be profitably grown in such areas.
Since, vegetable production is labor intensive it could engage landless and unskilled labor force in the rural areas. Furthermore, small farms are inefficient for growing crops such as wheat, cotton, sugarcane and rice, since a lot of money resource is prerequisite for growing these crops. With such circumstances, crops that are short duration and fetch high returns are suitable for such farms [3]. Therefore vegetables can be safely selected for not only for high returns but these are also a cheap source of essential nutrients. Review of literature showed that a lot of research has been carried out on different aspects of major food crops in Pakistan and vegetables and minor crops are ignored. If the research has been done it is not published at least. So it is unavoidable to improve our cabbage and cauliflower cultivars for high yield and required traits.
The traditional breeding method for the genetic improvement of cultivar has its own limitations as it requires years to complete a selection cycle. As an alternative, genetic transformation can be employed for development of required traits and resistance against biotic and abiotic stresses. The present research was designed to optimize the growth conditions to raise and maintain the disease free stock of frequently cultivated varieties of cabbage and cauliflower; later to be used in experimentation for genetic improvement of varieties at Centre of Excellence in Molecular Biology (CEMB), Lahore.
Methods
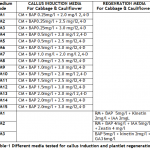
Conditions for in vitro mass production of cabbage and cauliflower were optimized. For this purpose two varieties of cauliflower, Chillout and White Shot, and one variety of cabbage, Saint, were selected. The F1 hybrid seeds of all the three cultivars were purchased from Sunny Seeds, 92-Karim Block Allama Iqbal Town, Lahore – Pakistan. The seeds were surface sterilized using 0.1% Mercuric chloride solution in distilled water. The seeds were soaked in this solution for 5 minutes and washed thrice with autoclaved distilled water under aseptic condition. After third washing seeds were kept in autoclaved distilled water for five minutes and then dried on autoclaved pieces of Whatman filter paper in laminar airflow cabinet. For germination, seeds were placed on moist paper in disposable Petri plates; plates were sealed with parafilm and incubated in dark at 20 ± 2°C. The hypocotyls of 5-7 days old seedlings were used as explant for callogenesis studies. The callus induction medium (CM) contained 4.43g/l MS basal medium (Sigma M5519), 3.0% sucrose, 0.3% gelling source (Phytagel; Sigma batch# 076K0168) and hormonal supplementations. Different concentrations of 2,4-D and BAP were used in combination to study callogenesis in cabbage and cauliflower. These concentrations of 2,4-D and BAP in media are given in Table-1. The pH of medium was adjusted between 5.5 and 5.7. The callus cultures were kept at 22±2° C under complete darkness to avoid browning of tissue due to phenolic compounds. When whole of the inoculated explant tissues proliferated into callus tissues, onwards the callus cultures were kept in 16 hours light period for production of chlorophyll in the cells. For regeneration experiments growth medium (RM) contained 4.43g/l MS, 3.0% sucrose, 0.3% phytagel, 150mg/l Myoinositol (Merck 0100), Adinine Sulphate (Merck), Thiamin HCl (Merck). Different concentrations of plant growth hormones tested for efficient regeneration of calli are given in Table-1. Cultures were kept in light for 16/24 hours at 22±2° C. For rooting of regenerated shoots, MS basal medium was used without any hormonal supplementation (zero). After the inoculation the cultures were regularly shifted to fresh media of same composition, for respective treatment, after every 15 days.
Suitability of a treatment was evaluated from percentage of cultures showing callus induction and plantlet regeneration response on that specific treatment in callogenesis regeneration studies respectively.
Results
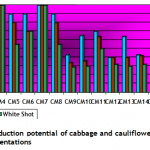
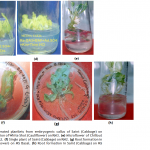
Callogenesis: To study the callus induction response of different cultivars, fifteen different callus induction media were examined. Hypocotyls explants of all three cultivars (Chillout and White Shot of cauliflower, and Saint of cabbage) were simultaneously cultured on all callus induction media given in Table-1. Generally within two weeks of first inoculums, the pale yellow color of explants tissue turned greenish along with increase in length approximately up to double of its original length. Then with passing days whole of the explants tissue turned into a fresh yellow colored mass of embryogenic callus. Figure-1(a) shows the initiation of callus at the edges of explants in 7 days old cultures on CM4 (CM + BAP 0.5mg/l + 2.0 mg/l 2,4-D) medium. After 20 days of inoculation, the percentage response of cultivars for callus induction was calculated in all media. (Figure-1(b) shows 27 days older callus of saint in CM1 (CM + BAP 0.25mg/l + 2.0 mg/l 2,4-D). Tabulated data helped to infer that CM1, CM3, CM6 and CM9 media were most effective for callus induction as all of the explant tissues (of all three cultivars) cultured on these media produced very viable and embryogenic calli (100% callus induction, Figure-2). Tabulated observations (Figure-2) expressed the fact that high concentration of 2,4-D (an auxin) in medium favors the repeated cell division and formation of callus and found better if used in ratio of 2:1 (at least) with auxin, respectively. After callus formed, higher BAP (cytokinin) was essential for plantlet regeneration as higher auxin caused increase in size of callus only and no regeneration or very little was observed. It was observed that cauliflower had more reproducibility potential in vitro condition for production of callus as compared to cabbage. Furthermore, Chillout variety of cauliflower showed better callus induction response over White Shot. Later observations showed that although callus induction was slower in White Shot its callus produced plenty of shoots and its shoots grew longer than Chillout.
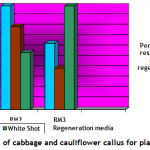
Plantlet regeneration: Embryogenic calli of each cultivar were shifted to three different compositions (Table-1) of growth media to study shoot regeneration response. For evaluation of regeneration potential Kinetin, Zeatin, Gibrellic acid-3, and Indole acetic acid were found good in combination with benzyl amino purine. Percentage response of cultivars on different plant regeneration media for in-vitro shoot regeneration was evaluated after fifteen weeks. Different cultivars showed different response on a specific supplemented medium. Saint (Cabbage) showed 100 percent plant regeneration i-e. all cultured calli produced plantlets on RM2 medium having BAP (4mg/l), IAA (5mg/l) and Zeatin (4 mg/l), (Figure-1 c & Figure-3). Similarly Chillout (Cauliflower) regenerated plantlets in maximum calli (80%) when shifted to RM1 and 100% cultures of White Shot (Cauliflower) produced plantlets on RM3 (Figure-1 d & Figure-3: it was found that for efficient plant regeneration from callus, higher concentration of cytokinin (higher than auxin) was essential to obtain good regeneration response of callus).
Rhizogenesis: The regenerated shoots were transferred to root forming media. Full strength MS media without any hormone (basal medium) was used for root induction. It was observed that examined varieties of cabbage and cauliflower had sufficient endo-auxin level and no supplementations of auxin were required for rooting of regenerated shoots. Figure-1 g & h show root formation in White Shot (Cauliflower) and Saint (Cabbage), respectively, immediately after transfer of cultures on to rooting medium.
Discussion
Many other researchers have also used different concentrations of plant hormones in growth medium for mass propagation of Brassica olerasea varieties. For instance, Rihan et al (2011) different concentrations of IAA, IBA, NAA and Kinetin are used in an effective protocol for the mass production of cauliflower microshoots using the meristematic layer of cauliflower curd [4]. He cultivated Explants in S23 liquid medium (4.4 g L-1 MS (Murashige and Skoog) and 3% v/w sucrose) supplemented with several combinations of plant growth regulators (PGRs) including 1 and 2 mg L-1 of Kinetin in combination with three types of auxins (indole butyric acid (IBA), Naphthaleneacetic acid (NAA) and Indole-3-acetic acid (IAA), each at 1 and 2 mg L-1 concentration. The use of 2 mg L-1 Kinetin and 1 mg L-1 IBA gave the best results in terms of its effects on explant induction. Some researchers obtained rapid shoot multiplication of transgenic Brassica oleracea (broccoli) at MS supplemented with different concentrations of BAP and NAA [5]. In one study, hypocotyls explants of Brassica oleracea (broccoli) were used to evaluate the callus induction and plant regeneration potential [6].
Dunwell (1981) successfully obtained adventitious buds and subsequently entire plants from excised leaf discs of three Brassica species, B. oleracea, B. napus, and B. campestris on MS media with specific combinations of 6-benzylaminopurine (BAP) and α-naphthylacetic acid (NAA) [7]. He found that each species required a particular hormone concentration for optimum growth and differentiation. Back in 1984, Lazzeri and Dunwell used hypocotyl for in vitro production of Brassica oleracea [8].
In conclusion, primarily the presented research was designed to raise the disease free stock of frequently cultivated varieties of cabbage and cauliflower to be used in experimentation for genetic improvement of varieties. But, results effectively concluded that these techniques can be used to produce in vitro bulk of plants on commercial scale in a very brief time span.
Figure 1(a) and 1(b) Table 1 Figure 1(c), 1(d), 1(e), 1(f), 1(g) and 1(h)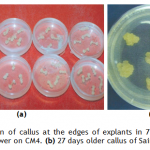
References
- Campbell B, Han DY, Triggs CM, Fraser AG, Ferguson LR. Brassicaceae: Nutrient analysis and investigation of tolerability in people with Crohn’s disease in a New Zealand study. Funct Foods Health Dis, (2012); 2460-486.
- Shah R, Ahmed S, Poswal A. Population dynamics of insect pests, parasitoids and predators in cabbage and cauliflower agro-ecosystems. Journal of Entomological Research, (2013); 37(2): 129-137.
- Bakhsh K, Akram W, Raza MA, Hassan I. Determination of factors affecting cauliflower yield in Punjab, Pakistan. Int J Agric Biol, (2004); 61056-1058.
- Rihan HZ, Al-Issawi M, Burchett S, Fuller MP. Encapsulation of cauliflower (Brassica oleracea var botrytis) microshoots as artificial seeds and their conversion and growth in commercial substrates. Plant Cell, Tissue and Organ Culture (PCTOC), (2011); 107(2): 243-250.
- Cao J, Earle E. Transgene expression in broccoli (Brassica oleracea var. italica) clones propagated in vitro via leaf explants. Plant cell reports, (2003); 21(8): 789-796.
- Kim JH, Botella J. Callus induction and plant regeneration from broccoli (Brassica oleracea var. italica) for transformation. Journal of Plant Biology, (2002); 45(3): 177-181.
- Dunwell J. In vitro regeneration from excised leaf discs of three Brassica species. Journal of Experimental Botany, (1981); 32(4): 789-799.
- Lazzeri P, Dunwell J. In vitro shoot regeneration from seedling root segments of Brassica oleracea and Brassica napus cultivars. Annals of botany, (1984); 54(3): 341-350.