Full Length Research Article
Therapeutic potential of stem cells derived factor-1 alpha (SDF-1α) for skin burn injuries
Nadia Wajid*,1, Azib Ali1, Fatima Ali1, Noreen Latief2, Aamer Qazi1
Adv. life sci., vol. 6, no. 4, pp. 139-146, August 2019
*- Corresponding Author: Nadia Wajid (Email: nadia.wajid@imbb.uol.edu.pk)
Authors' Affiliations
2. University of the Punjab, Pakistan
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Stem cells derived factor-1 alpha (SDF-1α) enhances the migration of bone marrow cells towards wound hence improve the healing process. The present study focuses on the therapeutic potential of SDF-1α and bone marrow derived cells from rats after SDF-1α treatment for skin burn.
Methods: SD rats were given thermal skin burn. Animals were divided into three groups i.e. SDF (S) with subcutaneous SDF-1α treatment after burn, Burn (B) and control (C). After seven days of treatment rats were sacrificed and skin, liver, heart, kidneys and blood were taken for histological and serological analysis. CBC, serum electrolytes, liver functions tests, renal functions tests, lipid profile, blood glucose as well as ELISA for VEGF, CRP and oxidative stress were performed. Cells were extracted from bone marrow and were transplanted in the burnt animals either directly or after culturing (Bone marrow stromal cells; BMSCs).
Results: A prominent role of SDF-1α on skin thermal wound healing was observed. Importantly the treatment has no significant impact on vital organs like liver, heart and kidney as well as physiological parameters. Lowered inflammation and enhanced angiogenesis was observed by ELISA for CRP and VEGF. BMSCs from SDF group showed higher growth (viability and proliferation), lowered cell death (LDH release), high angiogenesis (VEGF) and decreased oxidative stress. These BMSCs healed the burnt skin better than whole bone marrow of the same group after transplantation.
Conclusion: It may be concluded that SDF-1α can be a potential therapeutic agent for thermal skin burns and BMSCs after the treatment get higher growth and healing potential.
Keywords: Skin; Burn; CXCR-4; SDF-1α; BMSCs
Human skin is capable to perform extensive range of tasks including awareness, regulation of water and temperature, and makes a shield which acts as barrier. When the skin is disturbed during injury as in the case of critical burn, wounds or chronic wounds such as pressure and leg ulcers, the skin needs immediate healing so as to facilitate redevelopment and repair. Burn wounds are instigated by damage to the skin due to heat, chemical, infection, electric shocks or radiation [1]. Skin burns are among the 15 most prevalent ailments globally with approximately 300,000 annual deaths [2].
Healing of wounds involves interaction between signaling cascade and cells’ directly or through mediators. Coagulation, inflammation, angiogenesis, epithelialization, formation of granulation tissue, matrix and scar formation are the events involved in wound healing which are facilitated by chemokines and cytokines. These agents are the target for development of therapeutics for skin burn wound healing. The chemokines such as CXCL1-3, [3-8] as well as their receptors (CXCR1-2) are present in normal skin tissues but are elevated during the process of wound healing. SDF-1 SDF-1α is a chemokine mutually exclusive with the CXCR4 receptor found on BMSCs [3]. In normal wound healing, cellular migration of bone marrow derived stem cells from blood to injured tissue is facilitated by the SDF-1/CXCR4 pathway [4]. SDF-1/CXCR4 pathway is involved in the inflammatory, proliferative phase and migration [5]. SDF-1 expression in tissues is considered significant in mobilization as well as homing of BMSCs to injury sites. Hence for repairing of a damaged tissue SDF-1α in high concentration is required at the site of injury.[3]. It has been suggested that enhancement of CXCL1-2/CXCR4 axis would be a therapeutic strategy for burn wounds.5 Several studies have demonstrated that application of SDF-1α at wound sites increases the wound healing [6-9].
Based on the above literature we designed our study to evaluate the role of subcutaneous delivery of SDF-1α at injury site in skin burn model of rat. Therapeutic potential of whole bone marrow as well as bone marrow derived mesenchymal stem cells of rats after treatments were analyzed for skin burn injuries.
Rat Model of Skin Burn
The animals used in the experiment were treated according to the Instructions of the institutional ethical committee. Male rats weighing approximately 250-300g were selected for the experiment. Rats were anesthetized by giving 50mg/kg ketamine and xylazine 30mg intraperitoneally. Hair were removed and wet thermal injury was induced by boiling water for one minute at 15cm2 area.
Treatment
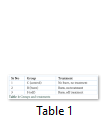
Rats without burn wound were nominated as control group, with only burn injury as burn while rats treated with SDF-1α in burn wound intra dermally were designated as SDF group. Treatment groups are mentioned in Table 1. All animals were kept for seven days in regulated environment and then sacrificed to collect blood, bone marrow and skin.
Transplantation of whole bone marrow
Bone marrow of each group was aseptically handled to obtain 106 cells which were transplanted in the burnt skin while keeping unburned controls, burned control and sham transplanted groups with only normal saline treatment. Transplantation was performed after two days of injury while blood and bone marrow were collected after ten days. Treatment groups are mentioned in Table 2.
Transplantation of bone marrow stromal Cells
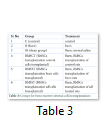
Bone marrow stromal cells (BMSCs) were cultured in low glucose DMEM till passage three. BMSCs of each group were then detached and 106 cells were transplanted in the burnt skin while keeping unburned controls, burned control and sham transplanted groups with only normal saline treatment. Transplantation was performed after two days of injury while blood and bone marrow were collected after ten days. Treatment groups are mentioned in Table 3.
Histological Analysis
Skin, heart, liver and kidney from all experimental groups were fixed in 10% formalin, dehydrated using ethanol grades (70%, 80%, 90%and 100%) and embedded in molten wax for 1 hour to prepare molds which were then cut in 5 μm thick sections with microtome. For hematoxylin and eosin (H & E) staining wax was removed from tissue sections using xylene. Rehydration was performed using alcohol grades (100%, 90%, 80% and 70% respectively) Sections were stained with hematoxylin (Sigma Aldrich, USA) and eosin solutions for 2 minutes each and mounted with Cytoseal (Richard-Allan Scientific, Thermo Fisher). Images were captured using EVOS FLoid cell imaging station (Life Technologies, USA).
LDH Assay
LDH assay was performed according to the manufacturer’s instructions (Roche). The reaction mixture contained reagent 1 (100μl), serum sample 4 μl, SR 20 μl, diluent 14 μl, and water to make total volume 138 μl and its absorbance was taken at 340/659nm.
Triglycerides; Cholesterol; Serum Electrolytes Estimation and Complete Blood Count
All these assays were performed commercially in The University of Lahore diagnostic labs.
Solid Phase Sandwich ELISA
Solid phase sandwich ELISA was performed for VEGF A, CRP, SDF, and IGF according to the protocol previously described by us [11].
Total Protein Estimation
Colorimetric kit-based method was performed according to the manufacturer’s (Roche) instructions.
Urea Estimation
This assay was based on kinetic colorimetric method. The reaction mixture contained reagent (Roche) 50 μl, serum sample 2 μl and water 193μl to make total volume 245 μl and its absorbance was taken at 340/409 nm. In this test urea was hydrolyzed by urease to form ammonia and carbonate. Test was done on cobas c 111.
Creatinine Estimation
This assay was performed by using Roche kit based on kinetic colorimetric method. The reaction mixture contained reagent 1 (13 μl), serum sample 10 μl, SR 17 μl, and diluents water 107 μl to make total volume 147 μl. Sample absorbance was taken at 512/583 nm. Test has been done on cobas c 111.
Culturing of BMSCs
BMSCs were isolated from tibias and femurs of rats from all experimental groups and cultured according to procedure reported by us [11].
Growth of BMSCs
Growth of cells was evaluated by cells’ viability and proliferation assay. For viability assay cells were stained with crystal violet (Invitrogen Inc., USA) and lysed with dimethylsulfoxide (DMSO) (Invitrogen Inc., USA) to compare colour taken by BMSCs of different groups as measure of viability of cells. Absorbance was taken at 540 nm. Proliferative potential of BMSCs was measured by using MTT (4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide) solution. Intensity of purple color taken by BMSCs after incubation in MTT solution was measured by taking absorbance at 570 nm.
Estimation of Oxidative Stress
Amount of reduced glutathione (GSH), activity of superoxide dismutase (SOD) and catalase (CAT) and concentration of malondialdehyde (MDA) in cell culture medium was estimated according to the method as reported earlier by Beutler et al. [10], Wajid et al. [11] and Ohkawa et al. [12].
Statistical Analysis
Total 39 animals were used in the study to perform experiments in triplicate. Statistical analyses were performed using GraphPad Prism 5 software (GraphPad, San Diego, CA). One-way ANOVA was used for comparison between different groups while keeping P<0.05 as standard for estimation of significance.
Effect of SDF1α treatment
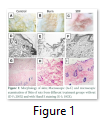
Skin: The untreated skin without thermal burn injury reflect clear damage to epidermis and dermis indicating second degree burn. While the subcutaneous injection of SDF1α improved the healing of second-degree burn generated in parallel (Fig. 1).
Physiological Parameters: Body weight of the rats from all groups was taken before treatment and after seven days i.e. before sacrifice. It was found that rats with burn injury showed reduction in weight (-4.000±14 g) as compared to control (6.000±4 g) which was recovered due to SDF-1α treatment (4.000±7 g). No significant difference was observed in the body temperature of rats due to burn (96.00±0.00 °C) or SDF-1α (975±0.95°C) treatment as compared to controls (95.33±0.57°C). Serum electrolytes did not vary significantly in all experimental groups. SDF-1α treatment and skin burn induced no significant difference on lipid profile (Fig. 2).
Complete Blood Count: Total blood count was performed for all treatment groups which indicate that there was no significant change in total leukocyte count of the groups after seven days of injury and treatment. The profile of thrombocytes indicated that all parameters including RBCs number (RBC), hemoglobin (HGB) levels, mega corpuscular volume (MCV), mega corpuscular hemoglobin (MCH), and mega corpuscular hemoglobin concentration (MCHC) had no difference among different treatment groups. Interestingly, the platelet profile had some significant differences i.e. a significant decrease in platelets distribution width (PDW), (MPV) and (P-LCR) while no significant difference was observed in platelets count (PLT) and (PCT) (Fig. 1S).
Cytotoxicity and Inflammation
Lactate dehydrogenase (LDH) assay was performed to assess the cytotoxicity in the three treatment groups and as expected LDH was higher in burn injury group (1519±361.3 U/L) as compared to control group (1105±358.7 U/L) while it was reduced in SDF-1α treated group (705.8±335.7 U/L). CRP is an indicator of inflammation which was significantly elevated in burn injury group (1.564±0.061) as compared to control (1.259±0.088 mg/L) and reduced to normal level in SDF-1α treated group (1.361±0.110 mg/L). (Fig. 2)
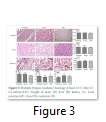
Multiple Organ Analysis: Internal organs such as heart, after burn and treatment, was analysed by histological analysis and weight of the organ was taken to assess the edema which revealed no significant change. Liver from all experimental groups were excised and weighed which revealed no statistically significant difference in all groups. Histological staining also did not show any deformation. Total protein analysis was performed to assess the hepatic functions in all treatment groups and it was observed that burn had no change (6.025±1.100 mg/dl) as compared to control (6.029±1.050 mg/dl) while SDF-1α had reduced total protein (756±0.5126 mg/dl) but it was not significant. Kidneys of rats of all treatment groups showed normal histology. The concentration of urea and creatinine was not changed significantly in all treatment groups indicating normal kidney functions (Fig. 3).
Angiogenesis
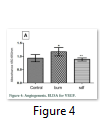
Angiogenesis was evaluated by sandwich ELISA for VEGF. It was found that VEGF increased significantly in burn injury group (1.178±0.1544) while it is reduced in SDF-1α treated group (0.9400±0.1354 units) and was equivalent to normal animals (0.8913±0.05502) (Fig 4).
Healing Potential of Whole Bone Marrow from treatment Groups
Skin: Whole bone marrow from SDF-1α treated animals transplanted in the thermally injured skin of rats showed the best wound healing with minimum wound area while the cells from whole bone marrow of control and burn animals also improved healing process better than those of burn as well as sham treated rats (Fig. 5).
Angiogenesis; ELISA for VEGF
Cytotoxicity: Serum from burn and sham transplanted rats had high level of LDH which was reduced in BMCT and BMBT group while BMST group indicated a significant reduction in LDH level almost equivalent to C (Fig. 5).
Characteristics of BMSCs
Stromal cells isolated from the bone marrow of rats of the study groups were initially analyzed in vitro for cells’ viability, proliferation, angiogenic ability, and chemokine (SDF) released then they were transplanted into the skin of burnt animals to analyze their healing potential. Cells’ Growth: It was observed that BMSCs of SDF-1α treated animals exhibited improved viability and proliferation as compared to burn and control animals (Fig. 6).
Angiogenesis: Similarly, VEGF was also highly released by BMSCs of SDF and burn rats as compared control (Fig. 6).
Chemokines Level: SDF and IGF were released significantly high by BMSCs of SDF rats as compared to control and burn (Fig. 6).
Oxidative stress: BMSCs isolated from the burn group expressed high activity of antioxidant enzymes GSH and SOD as compared to the BMSCs of SDF-1α and control. MDA level was also observed to be increased significantly in burn group BMSCs in comparison to SDF-1α treated and control group (Fig. 6).
Healing Potential of BMSCs from treatment Groups
Skin: Wound closure and clear skin was observed in rats transplanted with BMSCs from SDF group BMSCT and BMSBT groups also showed improved wound healing as compared to C, B and Sh groups (Fig. 7).
Cytotoxicity: Serum from burn and sham transplanted rats had high level of LDH which was reduced in BMSCT and BMSBT group while BMSST group (1011±133.6 U/L) indicated a significant reduction in LDH level almost equivalent to BMSCs (Fig. 7).
Figures & Tables
Scald injuries presents an economic burden globally and have a long recovery period [2]. Present therapies are either expensive or less effective so it is necessary to find the effective low-cost therapies for the deep skin burns through tissue regeneration. Skin wounds are healed by a balanced combination of multiple biological and molecular events including cell proliferation and migration of stem cells which promotes deposition of extracellular matrix as well as angiogenesis [13]. Present study focuses on the therapeutic potential of SDF-1α for the skin regeneration after deep burn as it promotes proliferation and migration of stem cells at the site of injury for repairing wound [3, 14]. After seven days of injury and SDF-1α treatment, blood and skin tissues were harvested for evaluation of inflammation, angiogenesis, paracrine factors, and skin healing.
The animals tolerated the scalds as no infection or death was recorded pre or post operatively. Macroscopic examination of skin revealed improved healing in the S group while microscopic analysis represented improved wound closure, tissue regeneration and re-epithelialization (Fig.1). Further, no change in physiological parameters was observed i.e. body temperature, serum cholesterol, triglycerides and electrolytes while body weight was reduced in burn injury group non-significantly. Severe skin burn causes a significant increase in LDH release in the burnt animals [15]. In the present study, LDH level was higher in burn injury group while it was reduced in S group (Fig. 2). It has been observed that thermal skin injury resulted in elevated plasma CRP levels in comparison with normal control rats [16]. In our study CRP was analyzed by ELISA to assess inflammation which was significantly elevated in burn injury group and reduced to normal level in S group confirming earlier studies (Fig. 2).
In the present study no significant effect on profiles of leukocytes and thrombocytes was observed in all treatment groups while the parameters of platelets were decreased significantly in SDF-1α treated animals. Platelets are small fragments of megakaryocyte cytoplasm. They play a fundamental role in primary and secondary hemostasis, as crucial reactions of the coagulation cascade occur on platelets phospholipid surface. The early phase of acute burn is characterized by a bleeding tendency, whereas the late phase is characterized by hypercoagulability. Although the platelet primary function is hemostatic regulation, they also act as inflammatory cells [17]. Our results showed a platelet count decrease in S group in relation to burn on the 7th day (Fig. 1S). It has been reported that inflammation resulted due to severe burn which then leads to oxidative stress and multiple organ failure [15]. In the present study, effects of burn and SDF-1α treatment were analyzed on liver, kidney and heart. Histology of all three organs did not reveal any significant anomaly (Fig. 3). Total protein analysis was performed to assess the hepatic functions in all treatment groups and it was observed that burn has no effect while SDF-1α has reduced the total protein but not significantly (Fig. 3). This observation may still be considered carefully for the therapeutic purposes. The concentration of urea and creatinine was not changed significantly in all treatment groups indicating normal kidney functions (Fig. 3). Skin regeneration requires development of new blood vessels or angiogenesis [18]. Burns lead to loss of blood vessels network in skin leading to reduced regeneration due to deficiency of oxygen and nutrients.2 Skin burn leads to tissue edema both locally and generally due to cell destruction and capillary leakage [19]. VEGF improves vascular permeability and angiogenesis. Serum levels of VEGF were reported to be increased immediately after burn and normalized after wound closure [20]. It was found in current study that VEGF increased significantly in burn injury group while it was reduced in S group, emphasizing the role of VEGF in wound closure (Fig. 4). It is reported that blood born inflammatory cells and bone marrow derived cells (BMDCs) are recruited to the injury site during the inflammatory phase of wound healing. Circulating BMDCs are even increased in the severe injury conditions and reconstitute the distant cutaneous wounds [21]. We transplanted whole bone marrow of rats from all three treatment groups allogenically to other burned animals to evaluate the repair potential. It was observed that BM transplantation improved the healing of burnt skin wound significantly than BM from SDF-1α treated animals. Data of LDH assay also supported the results (Fig. 5). Stem cells have potential to modulate acute and chronic wounds healing [22, 23, 24]. BMSCs are of considerable importance for burn wound repair due to their self-renewing and differentiation ability which has been used for regeneration of acute and chronic skin burn injuries in autologous and allogenic transplantation. BMSCs produce collagen, multiple growth factors and angiogenic factors in higher amounts which are required for skin wound healing. It is reported that BMSCs derived from patients express slow growth due to bone marrow suppression which is related to sepsis or toxicity of silver sulphadiazine [21]. In the present study no sepsis was observed and no difference was observed in growth of BMSCs from burn animals compared to normal animals as confirmed by cells viability by crystal violet staining, cells, proliferation by MTT assay and cytotoxicity by LDH assay. All these parameters were significantly improved in BMSCs derived from SDF treated rats. BMSCs were reported to synthesize higher amounts of growth factors including VEGF which has potential role in wound healing [21]. Our results show a significant increase in VEGF, IGF and SDF release by BMSCs from SDF treated rats hence enhanced repairing potential. For further evaluation of skin burn wound healing BMSCs from all groups were transplanted into burnt skin and BMSCs overall showed better healing while SDF treated animals showed the best healing and hair growth was even better than whole bone marrow transplantation (Fig. 6 & 7). In the present study, SDF-1α as therapeutic intervention to thermal skin burn injury has been studied. It was observed that subcutaneous injection of SDF-1α to the burn wound resulted in the improved healing with no harmful effect on vital organs like liver heart and kidneys as well as it induced no effect on complete blood count. Bone marrow derived from the treated animals had higher healing potential after transplantation. Furthermore, BMSCs of the treated animals have higher growth, increased growth factors release and low oxidative stress and increased wound healing potential after transplantation. This was even better than unfractionated bone marrow. It is concluded that SDF-1α is a better option for therapy of skin burn injury. Further studies are required on human subjects to evaluate the efficacy of SDF-1α as a therapeutic agent.
Acknowledgement
Thanks and gratitude to the efforts of all the patients who patiently and diligently helped us with this research, as well as from the company of Setareye Shakhab Qeshm who supported the research in providing the supplements needed.
The authors declare that there is no conflict of interest regarding the publication of this paper.
- Berthod F, Damour O. In vitro reconstructed skin models for wound coverage in deep burns. British Journal of Dermatology, (1997); 136:809–816.
- Giri P, Ebert S, Braumann UD, Kremer M, Giri S, et al. Skin regeneration in deep second-degree scald injuries either by infusion pumping or topical application of recombinant human erythropoietin gel. Drug Design, Development and Therapy, (2015); 9:2565-79.
- Tang T, Jiang H, Yu Y, He F, Ji S, et al. A new method of wound treatment: targeted therapy of skin wounds with reactive oxygen species-responsive nanoparticles containing SDF-1α. International Journal of Nanomedicine, (2015); 10: 6571-6585.
- Abkowitz JL, Robinson AE, Kale S, Long MW, Chen J. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood, (2003); 102:1249-1253.
- Hu C, Yong X, Li C, Lu M, Liu D, et al. CXCL12/CXCR4 axis promotes mesenchymal stem cell mobilization to burn wounds and contributes to wound repair. Journal of Surgical Research, (2013); 183:427-434.
- Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are Reversed by hyperoxia and SDF-1 alpha. Journal of Clinical Investigation, (2007); 117:1249–59.
- Rabbany SY, Pastore J, Yamamoto M, Miller T, Rafii S, et al. Continuous delivery of stromal cell derived factor-1 from alginate scaffolds accelerates wound healing. Cell Transplant, (2010); 19:399–408.
- Henderson PW, Singh SP, Krijgh DD, Yamamoto M, Rafii DC, et al. Stromal-derived factor-1 delivered via hydrogel drug-delivery vehicle accelerates wound healing in vivo. Wound Repair and Regeneration, (2011); 19:420–5.
- Sarkar A, Tatlidede S, Scherer SS, Orgill DP, Berthiaume F. Combination of stromal cell-derived factor- 1 and collagen-glycosaminoglycan scaffold delays contraction and accelerates re-epithelialization of dermal wounds in wild-type mice. Wound Repair and Regeneration, (2011); 19:71–9.
- Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. Journal of Laboratory and Clinical Medicine, (1963); 61:882-8.
- Wajid N, Naseem R, Anwar SS, Awan SJ, Ali M, et al. The effect of gestational diabetes on proliferation capacity and viability of human umbilical cord-derived stromal cells. Cell Tissue Bank, (2015); 16:389-97.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, (1979); 95:351-8.
- Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. A review of gene and stem cell therapy in cutaneous wound healing. Burns, (2009); 35:171-80.
- Guo R, Chai L, Chen L, Chen W, Ge Let al. Stromal cell-derived factor 1 (SDF-1) accelerated skin wound healing by promoting the migration and proliferation of epidermal stem cells. In Vitro Cellular & Developmental Biology, (2015); 51:578-85.
- Sener G, Sehirli AO, Gedik N, Dülger GA. Rosiglitazone, a PPAR-gamma ligand, protects against burn-induced oxidative injury of remote organs. Burns, (2007); 33:587-93.
- Bekyarova G, Tancheva S, Hristova M. Protective effect of melatonin against oxidative hepatic injury after experimental thermal trauma. Methods and Findings in Experimental and Clinical Pharmacology, (2009); 31:11-4.
- Marina Pavić, Lara Milevoj. Platelet count monitoring in burn patients. Biochemia Medica, (2007); 17:212-9.
- Hacker S, Mittermayr R, Nickl S, Haider T, Lebherz-Eichinger D, et al. Paracrine Factors from Irradiated Peripheral Blood Mononuclear Cells Improve Skin Regeneration and Angiogenesis in a Porcine Burn Model. Scientific Reports, (2016); 29:25168.
- Nielson CB, Duethman NC, Howard JM, Moncure M, Wood JG. Burns: Pathophysiology of Systemic Complications and Current Management. Journal of Burn Care & Research, (2017); 38:e469-e481.
- Infanger M, Schmidt O, Kossmehl P, Grad S, Ertel W, et al. CTAK/CCL27 accelerates skin regeneration via accumulation of bone marrow-derived keratinocytes. Stem Cells, (2006); 24:2810–2816.
- Chen M, Przyborowski M, Berthiaume F. Stem Cells for Skin Tissue Engineering and Wound Healing. Critical Reviews in Biomedical Engineering, (2009); 37: 399–421.
- Pang C, Ibrahim A, Bulstrode NW, Ferretti P. An overview of the therapeutic potential of regenerative medicine in cutaneous wound healing. International Wound Journal, (2007); 14:450-459.
- Aragona M, Dekoninck S, Rulands S, Lenglez S, Mascré G, et al. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nature Communications, (2017);1:14684.
- An Y, wei W, Jing H, Ming L, Liu S, et al. Bone marrow mesenchymal stem cell aggregate: an optimal cell therapy for full-layer cutaneous wound vascularization and regeneration. Scientific Reports, (2015); 23:17036.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0