Full Length Research Article
Effect of Propofol on ERK1/2 expression during day times in the rat hippocampus
Muhammad Rafiq1, Sajed Ali2,*
Adv. life sci., vol. 5, no. 3, pp. 130-134, May 2018
*- Corresponding Author: Sajed Ali (Email: sajed.ali@skt.umt.edu.pk)
Authors' Affiliations
2- University of Management and Technology, Sialkot Campus, Sialkot, Pakistan
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Anesthetics are responsible for imparting many effects on the central nervous system. We hypothesized that short duration anesthetics may have varied effects at different time of the day. Propofol (short duration anaesthesia) has showed circadian variation in loss of righting reflex during 24 hours of the day.
Methods: Characterization of the effects of propofol anaesthesia on ERK1/2 in hippocampus at two different times of the day was performed. Male rats received either an intra-peritoneal injection of 120 mg/kg of propofol to set short-duration anaesthesia state (20-30 minutes) or the equivalent amount of the lipidic solvent as controls. For both groups of animals, anaesthesia or control, the injections were performed either at ZT0 or at ZT12. One hour following the injection, the animals were euthanized; the brains were removed and immediately frozen. The amount of ERK1/2 was assessed by using immunohistochemistry on brain sections.
Results: The amount of ERK1/2 density was significantly decreased (P<0.05) in CA1, CA2 and CA3 areas of the hippocampus only when anaesthesia was performed at ZT12.
Conclusion: Our current results evidenced that the impact of propofol anaesthesia on hippocampus vary depending on the zeitgeber time.
Keywords: Extracellular Signal Regulated Kinase (ERK1/2), Immunohistochemistry, Hippocampus, Propofol, Zeitgeber Time
Propofol (2, 6-diisopropylphenol) is a highly effective IV anaesthetic and is widely used for general anaesthesia and for sedation with local anaesthesia [1,2]. Propofol produces rapid sedation with quick onset and offset and relatively few serious side effects (bradycardia, hypotension and dose-dependent respiratory depression). Propofol induced amnesia could be observed not only at low dose under its administration in both animal models and in human volunteers but also at recovery from anaesthesia [3,4]. The detailed mechanisms underlying the amnesic effect of propofol are still under examination. The amnesic effect of propofol is considered to be related to an inhibition of long term potentiation (LTP) at Schaffer collateral-commissural pathway to CA1 (Cornu Ammonis area) pyramidal cell synapses in the hippocampus [5-7]. Neuroscientists generally agree that the hippocampus has an important role in the formation of new memories about experienced events (episodic or autobiographical memory) and consolidation [8,9].
Several behavioral and cellular studies show that Extracellular Signal–Regulated Kinase 1/2 (ERK1/2) has a very important role in controlling Long-term potentiation (LTP) and memory formation in the adult brain [10-12]. ERK1/2 activation facilitates transcriptional events and as a result, it regulates distribution and functions of synaptic proteins to control many forms of synaptic plasticity, including LTP and memory in the hippocampus [5,6]. A concentration dependent increase was seen in both the membrane and cytosolic fractions of cortical cells in culture; these changes in ERK1/2 phosphorylation being induced by the binding of propofol to the GABAAR (Gama amino butyric acid type A receptor) complex [13]. Propofol has showed circadian effect for its hypnotic properties in rats [14]. Is there any effect of propofol on ERK1/2 at different zeitgeber times?
The goal of the study was to examine the effect of in vivo administration of propofol on ERK1/2 protein in the hippocampus at two different zeitgeber times; ZT0 (start of rest period) and ZT12 (start of activity period). For this, we used an ex-vivo technique, the immunostaining of ERK1/2 on brain sections collected one hour after the injection of propofol.
Male rats of 8 weeks (Long Evans, Charles River) were housed in 22-24°C and they were kept in 12:12 hours light–dark cycle with ad libitum for 15 days before the experiments. Propofol (Propofol Fresenius®1% manufactured by Fresenius Kabi, France) or the lipidic solvent Intralipid (Intralipid® manufactured by Fresenius Kabi, France) as a control were given intra peritoneally at the dose of 120mg/kg at ZT0 and ZT12. Animals were euthanized by exposure of carbon dioxide; the brains were quickly removed on ice and frozen in isopentane at -40°C. Sequential 25 mm-thick brain coronal sections were cut at -18°C on a cryostat at -3.14 coordinate from bregma according to the atlas of Paxinos et al. [15] (Fig 3).
Briefly, immunohistochemistry steps were carried out at room temperature (18-22°C) unless otherwise specified. All washes were carried out under gentle agitation for ten minutes and repeated three times in buffer, 0.02M TBS (Tris Buffer Saline). Incubations were carried out on flat slides in humid chambers and 400-500 µL of reagent was provided per each slide depending upon the surface area of the tissues. Tissues were fixed in 4% paraformaldehyde for seven minutes. After being fixed by the paraformaldehyde, slides were placed in 0.3% hydrogen peroxide diluted in 50 mL TBS for 15 minutes to quench endogenous peroxidases. Slides were incubated for 1 hour in 5% goat serum (Vector Lab Birlingame CA USA), then incubated in rabbit phosphorylated ERK1/2 antibody (Cell Signalling Technology California USA, Cat # 9102) to measure the total ERK1/2, in the dilution ratio1:500 overnight at 4°C. The secondary antibody was anti-rabbit made in goat (Vector Lab Birlingame CA USA, Cat # AP-1000). Sections were incubated with Avidine–Biotin peroxides complex (Vectastain Elite Kit, Vector Laboratories). Both Avidin-Biotins were used as 3 µL per mL of TBS 0.01%. Sections were stained with a chromagen DAB (3, 3- diaminobenzidine tetrahydrochloride by company SIGMA, Missouri USA), then sections were dehydrated and cover slipped.
The variable examined was the total ERK1/2 immunostaining per µm2. For each area, a global two way analysis of variance (ANOVA) was performed, between factors and time: ZT0 versus ZT12 and treatment: Intralipids versus Propofol. If a significant effect was observed, post hoc analyses were performed using a t-test with Bonferonni correction for multiple comparisons (Systat 8.0 software).
All the experiments on rats were performed as per approved guidelines and with the prior approval of the University Advanced Research Board, INSERM U666, University of Strasbourg, France.
These rats were divided into four groups on the basis of treatment and time of administration (n=6 per group). Following the injection of propofol, all animals lost their righting reflex in a 5-8 minutes delay. The mean time in minutes for ZT0 and ZT12 to recover the righting reflex following the injection of propofol was 28 and 35 respectively. One hour after the injection, all animals have recovered not only their righting reflex but also their spontaneous locomotion.
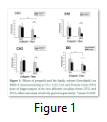
ANOVA in CA1 area of the hippocampus demonstrated that there are significant effects of treatment (F (1, 12) =25.88, P<10-4) and time (F (1.12) =7.48, P=0.017) with a significant interaction between treatment and time on the ERK1/2 immunostaining in CA1 area of the hippocampus (Fig 2). Indeed post hoc analysis revealed that there are significant differences only at ZT12, intralipid versus propofol (P=0.03) and for propofol, ZT0 versus ZT12 (P=0.05). General analysis of variance in CA2 area demonstrated that there are a significant effect of treatment (F (1, 12) =17.84, P<10-4) and no effect of time (F (1.13) =2.13, P=0.167) on the ERK1/2 immunostaining in CA2 area of the hippocampus. Indeed post hoc analysis revealed that there is a significant difference only at ZT12, intralipid versus propofol (P=0.03) and a marginal effect for ZT0 versus ZT12 in the propofol group (P=0.057). There is similar significant effects of treatment (F (1. 12) =9.61, P=0.003) and no effect of time (F (1.12) =1.232, =0.287) on the ERK1/2 immunostaining in CA3 area of the hippocampus. Indeed post hoc analysis revealed that there is a significant difference only at ZT12, intralipid versus propofol (P=0.014) and a marginal effect for ZT0 versus ZT12 in the propofol group (P=0.107). There is no significant effect of treatment (F (1, 12) =2.48, P=0.141) but a significant effect of time (F (1.12) =7.139, P=0.020) on the ERK1/2 immunostaining in DG area of the hippocampus (Fig.1).
Figures
In the present study, we observed the effect of propofol on ERK1/2 protein at two different zeitgeber times (ZT0 and ZT12). Our main results show remarkable decrease in the ERK1/2 protein at ZT12 in hippocampal areas (CA1, CA2 and CA3) without any change in the DG induced by propofol. However, we find significant difference at ZT0 and ZT12 for the control situation (Intralipids). No anesthetic effect at ZT12 may be due to the fact that DG has high density of neurons and is one of the brain structures with high rates of neurogenesis in the adult rats [16]. It is also observed that DG neurons born prenatally as well as postnatal, in contrast hippocampal neurons born prenatally [17]. There may anesthetic effect with large dose and or long duration anesthetics. Effect of zeitgeber time on general anesthetics can be of clinical importance, resulting in alteration of circadian and biological rhythms and might be responsible for sleep disorders as an increase in sleepiness, drowsiness and a decrease in vigilance [18].
Previous studies have shown that propofol may disturb the ERK1/2. A concentration-dependent increase of ERK-1/2 phosphorylation in the brain has been observed. This increase being blocked by a GABA antagonist, suggesting that the observed changes in ERK-1/2 phosphorylation were induced by the binding of propofol to the GABAAR complex [13]. Conversely, in cultured rat hippocampal neurons, propofol reduced the N-methyl-D-aspartate receptor (NMDAR) dependent activation of MAPK/ERK cascade [19]. NMDA receptors are phosphorylated at Ser897 and Ser896 positions and these sites for phosphorylation are blocked by propofol [20]. Therefore, it has been suggested that propofol may affect NMDAR–dependent ERK phosphorylation by affecting NMDA phosphorylation [7]. Our results obtained at recovery from anaesthesia are in accordance with those obtained in vivo by Kozinn et al [21]. It is an evidence of a long lasting inhibitory effect of propofol on ERK1/2 phosphorylation in hippocampus which is contrasting with the results obtained by Oscarson et al. [22] in brain cell culture. However, the methodology used in this last study differs largely from our study. Firstly, we examined the ERK1/2 contents at recovery from anesthesia, one hour after propofol administration while Orscarson examined ERK1/2 phosphorylation soon after the administration of propofol at concentration clinically relevant for an anesthesia state.
Decrease of hippocampal ERK1/2, appears to us important to understand the cellular mechanisms underlying the well described inhibitory effect of propofol on LTP and probably its amnesic effect. Indeed, a significant contribution of ERK1/2 phosphorylation to synaptic plasticity and memory formation in the adult brain has been established in behavioral as well as in cellular studies [11,23]. ERK1/2 is required for the induction of stable LTP while LTP inducing high frequency stimulation of schaffer- collateral inputs to area CA1 lead to the activation of ERK1/2 in CA1 [24]. Such a role of activation of ERK1/2 in memory has also been observed in vivo; the performance in a fear learning task in rodents being largely decreased when animals received Methyl ethyl ketone (MEK) inhibitors while, fear learning was associated with a significant activation of ERK1/2 in hippocampus in trained animals [25]. As mentioned above, propofol has amnesic effect at low dose, high anesthetic dose and at recovery from anesthesia [3,4]. The amnesic effect of propofol is considered to be related to its known inhibition of LTP at Schaffer collateral-commissural pathway to CA1 pyramidal cell synapses in the hippocampus [5-7]. From our results, we can suggest that a specific inhibitory effect of propofol on ERK1/2 in hippocampus might be one of the transcriptional targets to explain the inhibitory effect of propofol on hippocampal LTP.
Our results focusing on hippocampus evidenced clearly, and for the first time to our knowledge, that propofol has an inhibitory effect on ERK1/2 and that this inhibitory effect depends on the time of the day administration of propofol. Propofol is known to have inhibitory effect on hippocampal long term potentiation and memory. Our first results open a line of researches to understand the detailed cellular mechanisms involved in the memory effects of propofol, more particularly at recovery from anaesthesia.
The authors declare that there is no conflict of interest regarding the publication of this paper.
- Whitehead RA, Schwarz SK, Asiri YI, Fung T, Puil E, et al. The efficacy and safety of the novel peripheral analgesic isovaline as an adjuvant to propofol for general anesthesia and conscious sedation: a proof-of-principle study in mice. Anesthesia & Analgesia, (2015); 121(6): 1481-1487.
- Sebel PS, Lowdon JD. Propofol: a new intravenous anesthetic. Anesthesiology, (1989); 71(2): 260-277.
- Veselis RA. Memory function during anesthesia. Anesthesiology, (1999); 90(3): 648-650.
- Pain L, Angst MJ, LeGourrier L, Oberling P. Effect of a nonsedative dose of propofol on memory for aversively loaded information in rats. Anesthesiology, (2002); 97(2): 447-453.
- Wei H, Xiong W, Yang S, Zhou Q, Liang C, et al. Propofol facilitates the development of long-term depression (LTD) and impairs the maintenance of long-term potentiation (LTP) in the CA1 region of the hippocampus of anesthetized rats. Neuroscience letters, (2002); 324(3): 181-184.
- Takamatsu I, Sekiguchi M, Wada K, Sato T, Ozaki M. Propofol-mediated impairment of CA1 long-term potentiation in mouse hippocampal slices. Neuroscience letters, (2005); 389(3): 129-132.
- Fibuch EE, Wang JQ. Inhibition of the MAPK/ERK cascade: a potential transcription-dependent mechanism for the amnesic effect of anesthetic propofol. Neuroscience bulletin, (2007); 23(2): 119-124.
- Bonnici HM, Chadwick MJ, Lutti A, Hassabis D, Weiskopf N, et al. Detecting representations of recent and remote autobiographical memories in vmPFC and hippocampus. The journal of neuroscience, (2012); 32(47): 16982-16991.
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological review, (1992); 99(2): 195-231.
- Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, et al. Regulation and function of adult neurogenesis: from genes to cognition. Physiological reviews, (2014); 94(4): 991-1026.
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Current opinion in neurobiology, (2004); 14(3): 311-317.
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nature reviews Neuroscience, (2004); 5(3): 173-183.
- Oscarsson A, Juhas M, Sjolander A, Eintrei C. The effect of propofol on actin, ERK-1/2 and GABAA receptor content in neurones. Acta anaesthesiologica Scandinavica, (2007); 51(9): 1184-1189.
- Challet E, Gourmelen S, Pevet P, Oberling P, Pain L. Reciprocal relationships between general (Propofol) anesthesia and circadian time in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, (2007); 32(3): 728-735.
- Paxinos G, Ashwell KW, Tork I Atlas of the developing rat nervous system. 2013; 4:1-250. Academic Press.
- Cameron HA, Mckay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. Journal of Comparative Neurology, (2001); 435(4): 406-417.
- Miller MW. Generation of neurons in the rat dentate gyrus and hippocampus: effects of prenatal and postnatal treatment with ethanol. Alcoholism: Clinical and Experimental Research, (1995); 19(6): 1500-1509.
- Dispersyn G, Pain L, Challet E, Touitou Y. General anesthetics effects on circadian temporal structure: an update. Chronobiology international, (2008); 25(6): 835-850.
- Kozinn J, Mao L, Arora A, Yang L, Fibuch EE, et al. Inhibition of glutamatergic activation of extracellular signal-regulated protein kinases in hippocampal neurons by the intravenous anesthetic propofol. Anesthesiology, (2006); 105(6): 1182-1191.
- Kingston S, Mao L, Yang L, Arora A, Fibuch EE, et al. Propofol inhibits phosphorylation of N-methyl-D-aspartate receptor NR1 subunits in neurons. Anesthesiology, (2006); 104(4): 763-769.
- Kozinn J, Mao L, Arora A, Yang L, Fibuch EE, et al. Inhibition of glutamatergic activation of extracellular signal-regulated protein kinases in hippocampal neurons by the intravenous anesthetic propofol. Anesthesiology, (2006); 105(6): 1182-1191.
- Oscarsson A, Juhas M, Sjölander A, Eintrei C. The effect of propofol on actin, ERK‐1/2 and GABAA receptor content in neurones. Acta Anaesthesiologica Scandinavica, (2007); 51(9): 1184-1189.
- Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annual review of pharmacology and toxicology, (2002); 42(1): 135-163.
- English JD, Sweatt JD. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. The Journal of biological chemistry, (1996); 271(40): 24329-24332.
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nature neuroscience, (1998); 1(7): 602-609.
This work is licensed under a Creative Commons Attribution-Non Commercial 4.0 International License. To read the copy of this license please visit: https://creativecommons.org/licenses/by-nc/4.0