![]()
Micro Propagation in Advanced Vegetable Production: A Review
Shahid Javed Butt1*, Servet Varis2, Idrees Ahmad Nasir3, Suman Sheraz1, Azka Shahid4, Qurban Ali3
Adv. life sci., vol. 2, no. 2, pp. 48-57, February 2015
*-Corresponding Author:Shahid Javed Butt , (Email: sbutt61@yahoo.com)
Author Affiliations
2- Department of Horticulture, Faculty of Agriculture, Namik Kemal University, Tekirdag - Turkey
3- Center of Excellence in Molecular Biology, University of the Punjab Lahore - Pakistan
4- Department of Food Technology, PMAS-Arid Agriculture University Rawalpindi - Pakistan
Abstract![]()
Introduction
Methods
Discussion
Conclusion
References
Abstract
Micro propagation is a fast method of plant propagation that has a great potential to develop high quality as well as disease-free plants. Advancements in this field have led to the development of several techniques for rapid multiplication and improvement of a wide range of horticultural crops and their production systems. Micro propagation includes three types of vegetative propagation, 1) somatic embryogenesis, 2) adventitious shoot production and 3) axillary shoot production, which provide excellent opportunities for successful vegetable crops production. In Capsicum genera, annuum-chinense-frutescens complex have been made with white flowers and small yellow seeds. A number of experiments have also been done to demonstrate the influence of hypocotyl explant orientation on shoot bud induction in Capsicum spp. Sweet potato cuttings when grown in vitro with Florialite gives greater percentage of survival. Similarly, in vitro layering of gourds; a modification in micro propagation methods become more advantageous as it produces single shoot rather than multiple shoots. Likewise, an immobilized culture system of obtaining torpedo-stage embryos of carrots of uniform size and higher tuber yield with rapid multiplication rate for potato were also introduced by micro propagation techniques.
Key words: Micro propagation, Capsicum, Potato, In-vitro vegetables, Horticultural crops
Introduction
For the rapid multiplication of plants, micro-propagation is a refined and well adapted technique. Due to the fast speed of propagation it has a great profit-making potential, the high plant quality and the ability to produce disease-free plants. It is an art and science of plant propagation under in vitro conditions. The process of propagating plants includes several steps i.e. stock plant care, explant section and sterilization, media manipulation to obtain proliferation, rooting, acclimatization, and growing plants in field conditions. As it requires a sterile work-place, therefore micro propagation is usually done by hand which makes the process cost-intensive and monotonous for the workers. Micro propagation was first presented as a concept to the scientific community in 1960 by Morel for producing virus-free Cymbidiums. The necessary tools that made micro propagation a possibility, such as the development of media and an understanding of growth regulators, have been accessible only since the late 1950s. The actual establishment of profitable micro propagation as an industry became a reality during the 1970s and 1980s. But, in spite of the micro propagation industry being only 15 to 20 years old, considerable progress has been made in the culture of plant tissues and cells in vitro and in the experimental management of higher plant parts and cells as microorganisms [1]. Micro propagation consists of three types of vegetative propagation: (1) Somatic embryogenesis, in which structures are formed containing a shoot and root (2) Adventitious shoot production, comprising the meristem formation from callus tissue or directly from organized tissues i.e. epidermal or sub-epidermal cells, (3) Axillary shoot production where axillary buds and meristems give rise to shoots that are used to produce additional clonal shoots. Several examples of successful applications have been reported for a) meristem culture, b) organogenic micro propagation from undifferentiated tissues, cells or protoplast, c) zygotic embryo culture, d) somatic embryogenesis and e) gametic embryogenesis. Hu and Wang had successfully obtained meristem, shoot tips and bud cultures in some laboratory experiments [2]. Meristem cultures have been employed to eradicate virus infections in some asexually propagated species. Meristem and bud cultures have been used commercially for multiplication of some valuable genotypes. Zygotic embryo culture has been successfully employed to avoid post-fertilization, cross incompatibility during inter-specific transfer of genes among related plant species for many years.
Methods
Search strategy and selection criteria
A systematic scientific and technical literature review was compiled with the main themes of “Micro propagation”, “in vitro vegetables”, “vegetable production under in vitro conditions”, “applications of micro propagation in agriculture”, and “micro propagation for mass production of vegetables”. Literature found coherent with the review topic was rigorously screened for the inclusion of a wide range of reputed research articles published during 1980-2014. Figures, tabulated data and graphical representations were also included were applicable to make this review technically stronger.
Discussion
Applications of Biotechnology in Vegetable production
Capsicum peppers are most diverse vegetables grown all over the world. Capsicum annuum, is the most popular, genetically diverse, and economically important species. Capsicum chinense Jacq. (Habanero or Scotch Bonnet types) and Capsicum frutescens (Tabasco type) are important in Asia and are genetically in close resemblance with Capsicum annuum. Thus, the three species can be intercrossed to make up the annuum-chinense-frutescens complex (or annuum complex), having white flowers and small yellow seeds [3]. Finally, Capsicum pubescens R & P (purple flowers and black rough seeds) which represent separate taxons, and Capsicum baccatum var. pendulum (white flowers in yellow/green spots) have been grown abundantly and utilized for millennia [4]. Identification of numerous traits of interest among the Capsicum species has been enabled due to such diversity (e.g. resistance against pests and diseases). However, the uses of interspecific crosses for transferring these traits between Capsicum species is usually hampered by the incompatibility problems such as embryo abortion. Long breeding cycle is another challenge for Capsicum breeders (i.e. from sowing seeds to harvesting ripened fruits containing the seeds for the next generation), which are considerably longer than other fruit vegetables (e.g. tomato, eggplant, melon). Thus, isolation and in vitro culture of zygotic embryos is incredibly helpful in both situations. This technique was first reported in two Cruciferae vegetables i.e. Cochleira and Raphanus and since then it has been very useful not only for shortening the breeding cycles or enabling interspecific and intergeneric hybridizations, but also for overcoming the seed dormancy and sterility, thus obtaining valuable haploid materials for micro propagation [5-7]. As a whole, the knowledge of the responses of Capsicum zygotic embryos to in vitro culture is still relatively scarce. Therefore, to overcome the post-fertilization genetic barriers, it has been applied in a few unique Capsicum annuum × Capsicum frutescens and Capsicum annuum × Capsicum baccatum crosses [8,9]. Despite the interest of all these studies, the use of a higher genotypic diversity is still missing and, only embryos beyond torpedo stage were reported to provide satisfactory results as it is extremely difficult to produce plantlets from heart or globular embryos [8].
All these findings have confirmed that the developmental stage of embryos is another key factor for their responses to in vitro culture, besides the genotypes [11]. So, the stages at which embryo abortion in Capsicum species may occur in interspecific hybridizations is very much dependent on the genotypes that are involved in the crossing which showed contrast to the results from Yoon et al. [8]. Several examples are there in which embryos had to be rescued at the very early stages [7,12]. Therefore, further studies regarding globular and heart embryos are required to improve the responses to in vitro cultures. Moreover, it is commonly accepted that composition of the media used to grow plants in vitro also has a significant effect on the regeneration of Capsicum plant tissues [13]. Providing immature embryos to promote growth is considered an additional key factor with the appropriate level of carbohydrates, mainly sucrose, and minerals, mainly MS mineral solution [14,15]. By contrast, exogenously supplied hormones may possibly modify the ontogenic pattern of embryos or, even, provoke callus formation, while, in some conditions, low concentrations of supplied hormones had facilitated embryo cultures [16,17]. In addition, Capsicum peppers are considered very recalcitrant for in vitro cultures [17] and therefore, two media were reported from the experiments of Yoon et al., [8] and Hossain et al., [9]. Beside this information there is a lack of detailed studies about the response of immature Capsicum embryos to different media and their interactions with the different genotypes and developmental stages is not reported yet.
Sweet potato cuttings grown in vitro on Florialite
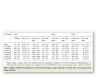
The growth and quality of plantlets in vitro are considerably influenced by nature of supporting material for rooting. Poor root development ultimately affects the shoot development as root and shoot development are inter-dependent. Agar which is a conventional gelling agent, has a lots of drawbacks such as lowering oxygen diffusion coefficient, negatively affecting the differentiation and development of tissues grown on cultures has limited adaptability to automation, inability to circulate the nutrient medium in the culture vessel, hard to clean the roots and the vessel before transplanting and significant differences in the sensitivity of the plant species to different agar brands. Use of various fibrous materials such as cellulose, rock wool and artificial soil such as vermiculite has been reported in enhancing the growth of plants in vitro. The supporting material, Florialite, is a mixture of paper pulp and vermiculite, has the ability to absorb up to 71% nutrient solution of its own volume [1]. The growth of the plantlets obtained from Florialite was greater than that of the vermiculite which indicates that the addition of paper pulp with vermiculite is the major reason for this greater growth. Moreover, the Florialite solid cubes makes it possible to automate the micro propagation and handling of material more conveniently and precisely compared with that of agar or vermiculite granules. Therefore, sweet potato cuttings were grown photoautotrophically in vitro in supporting material of either 100% vermiculite or vermiculite mixed with different concentrations of paper pulp. The use of vermiculite with 30% paper pulp was found to stimulate growth of leaves, stems and roots significantly, and any deviation from this proportion of paper pulp brought about a regular reduction in the root and shoot growth (Table 1). The difference is clear between the mixture of vermiculite and paper pulp treatments and the control in the development of the root system. The main adventitious roots gave rise to fine lateral roots for those plantlets that are grown in the mixture of vermiculite and paper pulp.
Micro propagation in gourds (Trichosanthes dioica Roxb.) through cuttings
Pointed gourds (Trichosanthes dioica Roxb.) from family Cucurbitaceae are dioecious perennial herbaceous vegetables. Fruits are the edible portion and are a good source of vitamin C and minerals [18]. This crop is propagated conventionally by shoot cuttings using 60-90 cm long segments from basal portion of the vines. Seed propagation in gourds is not desirable due to its cross pollinated nature, poor germination, slow seedlings growth and segregation of male and female plants [19]. As it is perennial crop, this characteristics of the crop provides an opportunity for cost effective micro propagation of superior and desirable clones. Only few cultivars of Trichosanthes dioica breed crop are reported. Two such highly productive female types `Swarna Alaukik' and `Swarna Rekha' that are released from the Central Horticultural Experiment Station (CHES), Ranchi (North India) and are in high demand and no adequate supply of the planting material is available. Media treatments that involve cytokinins and indole-3-acetic acid (IAA) with a view to induce multiple shoots gave poor responses together with callusing and differentiation. Different combinations of GA3 and BA resulted in multiple shoots along with poor elongation and high vitrification. Thus, in vitro layering was more advantageous than the multiple shoot production pathway, in which the micro cuttings produce a single shoot often coupled with rooting [20,21]. The significant feature is that a single medium formulation facilitated culture initiation, multiplication and rooting in all the genotypes. Further, the rooted stumps that are left after the sub-culturing of nodes could be used effectively for ex vitro establishment of plantlets.
Somatic embryogenesis in Carrots (Daucus carota subsp. Sativus)
Somatic embryogenesis is potentially one of the most efficient methods for plant micro propagation and in case of dicotyledonous culture, embryos at various developmental stages i.e. globular-, heart-, torpedo-, and cotyledonary-stage are frequently mixed in the suspension [22,23]. Additionally, the rate of formation of embryos in each developmental stage alters correspondingly during the culture period in carrots. Somatic embryos harvested at the torpedo-stage, have better performance of plant conversions. Therefore, several attempts have been made to develop certain methods that results in obtaining a large number of torpedo-stage embryos of carrots with satisfactory degree of homogeneity.
Two approaches are classified in this respect. First is to select torpedo-stage embryos from a heterogeneous embryo population of carrots. A few groups have been reported on automated selection systems of torpedo-stage embryos that are combined with image analysis systems (Fig. 1, 4, 5). Molle et al. [24] have developed an automated selection system based on filtration through a mesh that mechanically sieves torpedo-stage embryos. The second approach is to enhance synchronous development of embryos into torpedo-stage during the culture that aims at improving the culture method. Nadel et al. [25] showed that synchronization by addition of abscisic acid to the regeneration medium of somatic embryogenesis was promoted and Osuga et al. [26] proposed a method of improving synchronizing development into torpedo stage embryos by using inhibitory effect of high population density on embryos’ development. An immobilized culture system of obtaining torpedo-stage embryos of uniform size released from gel beads was developed [27].
These methods not only require high labor, but also involve a high risk of contamination during of embryo transfer into culture vessels. Jay et al. [29] reported that low medium pH 4.3 did not allow the development into torpedo- and plantlet-stage, thus suggesting that control of medium pH might be useful for synchronizing development into globular- and heart- stage embryos. Conversely, his report did not assess the development from heart-stage into plantlet-stage. Shimazu and Kurata [28] showed that low dissolved oxygen (DO) level (2.4 mg F’) in the culture media inhibited development of embryos into cotyledonary-stage, and thus increased the formation rate of torpedo-stage embryos within the cultures. DO levels can be controlled easily by changing the oxygen concentrations of the aeration. Therefore, synchronous cultures of somatic embryos were expected to be applicable to efficient production of torpedo-stage embryos by controlling DO level [30]. However, the development of embryos from torpedo-stage into cotyledonary-stage was halted so the development of globular and heart-stage embryos to later stages was also delayed by the low DO levels [31]. Resultantly, a remarkable number of globular and heart-stage embryos remained in the suspension when embryo were harvested at torpedo-stage. These results suggested that if there were a way to promote the development of globular and heart-stage embryos into the later stages, while simultaneously repressing torpedo-stage embryo development into cotyledonary-stage the formation rate of torpedo-stage embryos to total embryos of all developmental stages (FT) could be enhanced. It seems from the data on the embryo development that this could only be achieved by controlling DO in the suspension dynamically. The scientists proposed a dynamic DO control algorithm that enhances FT by promoting the development of early stage embryos into torpedo-stage embryos and repressing development of torpedo-stage embryos into late stages simultaneously [32]. This algorithm was based on information on the composition of embryo population (formation rate of embryos at each developmental stage to total embryos of all developmental stages) in the suspension that is assessed by monitoring all cultures. Since destructive sampling of somatic embryos has a risk of contaminating healthy cultures and is labor intensive, there is a need of a noninvasive monitoring method. Noninvasive monitoring by the image acquisition and analysis has been applied to automatically selecting normal somatic embryos from the suspension.
The monitoring in prescribed review study was also based on the noninvasive image acquisition of the embryo population during cultures. The pH of medium is one of the environmental factors that can be measured on-line very easily. It was also reported that changes in the pH of medium during somatic embryo culture showed characteristic variations, and these variations seemed to reflect the developmental phase and physiological state of the somatic embryos. Thus, measurement of medium pH could be a satisfactory way of monitoring carrot embryo development.
In vitro tuber yields and multiplication rates of potato
Potatoes are traditionally multiplied by tubers, with a multiplication rate of 6 to 10-fold annually. A major impediment to the introduction of new cultivars and to the production of high-quality tubers is the low production rate for tuber planting. Micro propagation can be a successful tool for enhancement of multiplication rates considerably. Under aseptic and controlled i.e. laboratory conditions, the rate of multiplication goes from 10 to 25-folds per 8 weeks, 8 to 84-fold per 40 days [33], and 4 to 7-fold per 3-5 weeks [34-36] have been reported by researchers. The desired quantity of plantlets can be produced in very short time by using this technique. However, temperature controlled chambers, well-equipped laboratories, mist equipment or greenhouse space [37] are required for rooting and hardening procedures as these are laborious and very time consuming. When large number of plantlets has to be produced then these factors might become limiting. In a study conducted by Levy [34], the remarkable capability of these plantlets to produce 16-25 tubers per plant was observed and the possibility of planting in vitro plantlets in the field was also shown. The study conducted by Wattimena et al. [37] and Wiersema et al. [38] showed the capability of in vitro plants to produce more but small sized tubers than plants grown from in vitro tubers. Moreover, transplantation of various plant species is a common procedure that is carried out by hand labor and also by various types of machines.
Morphogenesis in Capsicum annuum
Capsicum regeneration includes formation of profuse leafy structures instead of shoot bud, or shoot bud that do not elongates or induction of somatic embryos that fail to develop into a shoot [39,40]. Because of the difficulties in plant regeneration, applications of cell and molecular biology techniques for genetic improvement of Capsicum have been limited [41]. Although various reports on embryogenesis and organogenesis are available [13], none of them provide promising response in Capsicum annuum var. Arka Abhir (AA) and Arka Lohit (AL), which are two commercially important varieties of this species. This can be attributed to intra varietal differences in regenerations from different explants [42] and difficulties in elongation of shoot bud and somatic embryos reported in Capsicum annuum [10,40,43]. The effort reported here primarily addresses the role of polarity of explant, that are exogenously administered auxin transport inhibitor tri-iodo benzoic acid (TIBA), phloroglucinol (PG) along with benzyl adenine (BA) and IAA on direct adventitious shoot formation in genotypes of Capsicum annuum cv. Arka Abhir and Arka Lohit that are recalcitrant. Moreover, various studies were also carried out on the role of light in presence of exogenously fed GA3and AgNO3 in elongation of shoot buds.
An innovative in vitro regeneration protocol in tomato
Tomato is among the most important vegetables and widely used as raw or cooked. Beside a source of antioxidants, minerals, fibers and vitamins it is also an excellent sculpt system for genetic studies, fruit development and ripening process. Insects, pests and different pathogens can reduce tomato yield in the field. Microbial pathogens include lepidopteron, Helicoverpa armigera and common fruit borers which largely attack on fruit while Spodoptera litura destroy leaves. Incorporation of Bt genes in tomato has showed considerable resistance against lepidoterons. Tomato has served as an excellent model for plump fruit development and ripening. Well characterized ripening mutants, high density genetic maps, small genome size, short life cycle, efficient and stable transformation made tomato an excellent sculpt for studying viruses, development and fruit ripening process through genetic modification. Cold, heat and soil salinity are the main environmental factors that significantly affect the productivity and quality of tomato and other crops. Chilling, drought and salinity stress have been found interconnected in effecting water relations at cellular, tissue, organ and whole plant level leading to physiological, morphological biochemical and molecular changes. Numerous genetic modifications in tomato have been done [44-46].
The application of plant tissue culture pre supposes the establishment of proficient culture system, which consists of a competent genotype and explant source as well as optimal culture conditions. Most techniques for genetic regeneration depend on the use of plant growth regulators in complex and nearly empirical combinations modified to each particular situation. Progress of protocols independent of exogenous plant growth regulators could help standardize techniques for diverse species and cultivars, thereby, reducing problems of regeneration efficiency and elongation of regenerated and abnormal shoots.
Several protocols have been published for in vitro plant regeneration of tomato species. Methods formerly reported are tedious and time consuming, with variable efficiencies and high production costs. In all cases, regeneration systems implicated media containing growth regulators. A protocol for an efficient, rapid and high frequency plant regeneration method of normal tomato plants has been established which works independent of exogenous growth regulators in culture medium. It also reports the absence of morphological and chromosomal variations among regenerated plants.
In vitro development of Cauliflower synthetic seeds
The production of quality seeds is one of the main problems in cauliflower cultivation. Cauliflower, an open pollinated plant faces technical challenges to make in-bred lines with reliable self-incompatibility. Cabbage and cauliflower are two of numerous vegetables in the species Brassica oleracea of family Brassicaceae. These are grown, in Pakistan, for their edible value and are main cash crops of Pakistan. These are typically grown as a winter crop and sometimes also as summer vegetables. Vegetable production carries a valuable position in edible crops of Pakistan. Although the cabbage and cauliflower are among minor crops and are mostly grown in small farms even then Cauliflower is one of the most cultivated vegetable in the Punjab Province. Generally landless and poor farmers prefer to grow vegetables on small farms because vegetable production gives higher returns than cereals and other crops. As landless labor class spread through the rural areas therefore these crops can be profitably grown in such areas [47].
An improved seed biotechnology resulting in efficient and stable regeneration methodology is a heavy demand. Therefore an effective protocol for the production of cauliflower propagules was designed from fractionated and graded curd then these propagules were encapsulated in sodium alginate. The major objective was to form cauliflower synthetic seeds from an efficient callus induction protocol from hypocotyls for cell suspension.
The traditional breeding method for the genetic improvement of cultivar has some limitations as it requires years to complete a selection cycle. As an alternative, genetic transformations can be employed for development of required traits and resistance against biotic and abiotic stresses.
This cauliflower synthetic seed production from hypocotyl, its successful germination viability and regeneration into a complete plantlet is the first successful study. This study has the main objective of optimizing a simple, inexpensive and sophisticated method for cauliflower synthetic seeds production that would be viable as vegetative propagules that can be stored for longer period of time and consequently able to germinate to produce complete healthy and viable plants. Production of synthetic seeds seems to be a promising method in plant tissue culture industry of cauliflower.
Figures & Tables
Figure 1 Figure 2 Figure 3 Figure 4



Conclusion
It is concluded that the micro-propagation technique has a great profit-making potential, the high plant quality and the ability to produce disease-free plants. It should be used on large scale to develop new vegetable varieties and to increase the production of vegetables to full fill the requirement of growing world population.
References
- Afreen-Zobayed F, Zobayed S, Kubota C, Kozai T, Hasegawa O. A combination of vermiculite and paper pulp supporting material for the photoautotrophic micropropagation of sweet potato. Plant Science, (2000); 157(2): 225-231.
- Wong W. In vitro propagation of banana (Musa spp.): initiation, proliferation and development of shoot-tip cultures on defined media. Plant Cell, Tissue and Organ Culture, (1986); 6(2): 159-166.
- Rodríguez-Burruezo An, Kollmannsberger H, González-Mas MC, Nitz S, Fernando N. HS-SPME Comparative Analysis of Genotypic Diversity in the Volatile Fraction and Aroma-Contributing Compounds of Capsicum Fruits from the annuum− chinense− frutescens Complex. Journal of agricultural and food chemistry, (2010); 58(7): 4388-4400.
- Rodríguez-Burruezo A, Prohens J, Raigón M, Nuez F. Variation for bioactive compounds in ají (Capsicum baccatum L.) and rocoto (C. pubescens R. & P.) and implications for breeding. Euphytica, (2009); 170(1-2): 169-181.
- Tukey H. Artificial culture of sweet cherry embryos. Journal of Heredity, (1933); 24(1): 7-12.
- Acebedo M, Lavee S, Linan J, Troncoso A. In vitro germination of embryos for speeding up seedling development in olive breeding programmes. Scientia Horticulturae, (1997); 69(3): 207-215.
- LanZhuang C, Adachi T. Efficient hybridization between Lycopersicon esculentum and L. peruvianum via ‘embryo rescue’and in vitro propagation. Plant Breeding, (1996); 115(4): 251-256.
- Yoon JB, Yang DC, Do JW, Park HG. Overcoming two post-fertilization genetic barriers in interspecific hybridization between Capsicum annuum and C. baccatum for introgression of anthracnose resistance. Breeding Science, (2006); 56(1): 31-38.
- Hossain MA, Minam M, Nemoto K. Immature embryo culture and interspecific hybridization between Capsicum annuum L. and C. frutescens L. via embryo rescue. Japanese Journal of Tropical Agriculture, (2003); 47(1): 9-16.
- Manzur J, Penella C, Rodríguez-Burruezo A. Effect of the genotype, developmental stage and medium composition on the in vitro culture efficiency of immature zygotic embryos from genus Capsicum. Scientia Horticulturae, (2013); 161: 181-187.
- Kapila R, Sethi G. Genotype and age effect on in vitro embryo rescue of bread wheat x hexaploid triticale hybrids. Plant cell, tissue and organ culture, (1993); 35(3): 287-291.
- Barbano P, Topoleski L. Postfertilization hybrid seed failure in Lycopersicon esculentum+^ Lycopersicon peruvianum ovules. Journal of the American Society for Horticultural Science, (1984); 109(1): 95-100.
- Ochoa-Alejo N, Ramirez-Malagon R. In vitro chili pepper biotechnology. In vitro Cellular & Developmental Biology-Plant, (2001); 37(6): 701-729.
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum, (1962); 15(3): 473-497.
- Sharma D, Kaur R, Kumar K. Embryo rescue in plants—a review. Euphytica, (1996); 89(3): 325-337.
- Carman JG. Embryogenic cells in plant tissue cultures: occurrence and behavior. In vitro Cellular & Developmental Biology, (1990); 26(8): 746-753.
- Kothari S, Joshi A, Kachhwaha S, Ochoa-Alejo N. Chilli peppers—a review on tissue culture and transgenesis. Biotechnology Advances, (2010); 28(1): 35-48.
- Sharmila BG, Kumar G, Rajasekara PM. Cholesterol lowering activity of the aqueous fruit extract of Trichosanthes dioica Roxb. in normal and streptozotocin diabetic rats. Journal of Clinical Diagnostic Research, (2007); 1: 561-569.
- Wang H, Chen C, Sung J. Both warm water soaking and matriconditioning treatments enhance anti-oxidation of bitter gourd seeds germinated at sub-optimal temperature. Seed Science and Technology, (2003); 31(1): 47-56
- Rai GK, Singh M, Rai NP, Bhardwaj D, Kumar S. In vitro propagation of spine gourd (Momordica dioica Roxb.) and assessment of genetic fidelity of micropropagated plants using RAPD analysis. Physiology and Molecular Biology of Plants, (2012); 18(3): 273-280.
- Mythili J, Thomas P. Micropropagation of pointed gourd (Trichosanthes dioica Roxb.). Scientia Horticulturae, (1999); 79(1): 87-90.
- Schenk RU, Hildebrandt A. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Canadian Journal of Botany, (1972); 50(1): 199-204.
- Bornman CH. Micropropagation and somatic embryogenesis. Plant Breeding. (1993): pp. 246-260. Springer.
- Monnier M. Culture of zygotic embryos. In vitro embryogenesis in plants. (1995): pp. 117-153. Springer.
- Nadel B, Altman A, Ziv M. Regulation of somatic embryogenesis in celery cell suspensions. Plant Cell, Tissue and Organ Culture, (1990); 20(2): 119-124.
- Osuga K, Kamada H, Komamine A. Cell density is an important factor for synchronization of the late stage of somatic embryogenesis at high frequency. Plant Tissue Culture Letters, (1993); 10(2): 180-183.
- Suehara K-I, Kohketsu K, Uozumi N, Kobayashi T. Efficient production of celery embryos and plantlets released in culture of immobilized gel beads. Journal of Fermentation and Bioengineering, (1995); 79(6): 585-588
- Teruaki S, Kenji K. Improvement of Synchronization on Carrot Somatic Embryo Culture by Controlling Dissolved Oxygen Concentration. Environment Control in Biology (1999); 37(3): 179-184.
- Jay V, Genestier S, Courduroux J-C. Bioreactor studies of the effect of medium pH on carrot (Daucus carota L.) somatic embryogenesis. Plant Cell, Tissue and Organ Culture, (1994); 36(2): 205-209.
- Misra BB, Dey S. Culture of East Indian sandalwood tree somatic embryos in air-lift bioreactors for production of santalols, phenolics and arabinogalactan proteins. AoB Plants, (2013); 5plt025.
- Xie D, Hong Y. Regeneration of Acacia mangium through somatic embryogenesis. Plant Cell Reports, (2001); 20(1): 34-40.
- Kurata K, Shimazu T. Effects of dissolved oxygen concentration on somatic embryogenesis. Plan Tissue Culture Engineering, (2006). 6: 339-353.
- Marinus J. Some aspects of the in vitro multiplication of potatoes. Potato Research, (1983); 26: 85-86.
- Levy D, Izhar S, Fogelman E, Itzhak Y, Levy Y, et al. Multiplication rates of potato plantlets produced from tip culture and of tubers from this source grown in a screenhouse in the Golan. Hassadeh, (1983); 53934-938.
- Reust W, Dutoit J, Thomas D. La multiplication rapide des pommes de terre par le microbouturage. Revue suisse d'agriculture, (1985). 17(1): 11-18.
- Goodwin P, Kim Y, Adisarwanto T. Propagation of potato by shoot-tip culture. 1. Shoot multiplication. Potato Research, (1980); 23(1): 9-18.
- Wattimena G, McCown B, Weis G. Comparative field performance of potatoes from microculture. American Potato Journal, (1983); 60(1): 27-33.
- Wiersema S, Cabello R, Tovar P, Dodds J. Rapid seed multiplication by planting into beds micro tubers and in vitro plants. Potato Research, (1987); 30(1): 117-120.
- Hyde CL, Phillips GC. Silver nitrate promotes shoot development and plant regeneration of chile pepper (Capsicum annuum L.) via organogenesis. In vitro-Plant, (1996); 32(2): 72-80.
- Steinitz B, Küsek M, Tabib Y, Paran I, Zelcer A. Pepper (Capsicum annuum L.) regenerants obtained by direct somatic embryogenesis fail to develop a shoot. In vitro Cellular & Developmental Biology-Plant, (2003); 39(3): 296-303.
- Liu W, Parrott W, Hildebrand D, Collins G, Williams E. Agrobacterium induced gall formation in bell pepper (Capsicum annuum L.) and formation of shoot-like structures expressing introduced genes. Plant Cell Reports, (1990); 9(7): 360-364.
- Jacobs J, Stephens C. Factors affecting the regeneration of pepper (Capsicum annuum L.). HortScience, (1990); 25(9): 1120-1120.
- Shimazu T, Kurata K. Dynamic dissolved oxygen concentration control for enhancing the formation rate of torpedo-stage embryos in carrot somatic embryo culture. Journal of Bioscience and Bioengineering, (2003); 95(4): 384-390.
- Kumar V, Sharma A, Prasad BCN, Gururaj HB, Giridhar P, et al. Direct shoot bud induction and plant regeneration in Capsicum frutescens Mill.: influence of polyamines and polarity. Acta Physiologiae Plantarum, (2007); 29(1): 11-18.
- Moore S, Vrebalov J, Payton P, Giovannoni J. Use of genomics tools to isolate key ripening genes and analyse fruit maturation in tomato. Journal of Experimental Botany, (2002); 53(377): 2023-2030.
- Knight S, Rogers R, Smith M, Sporaer L. Effects of NaCl salinity on miniature dwarf tomato ‘Micro‐Tom’: I. Growth analyses and nutrient composition 1. Journal of Plant Nutrition, (1992); 15(11): 2315-2327.
- Shah R, Ahmed S, Poswal A. Population dynamics of insect pests, parasitoids and predators in cabbage and cauliflower agro-ecosystems. Journal of Entomological Research, (2013); 37(2): 129-137.