Characterization and efficiency assessment of PGPR for enhancement of rice (Oryza sativa L.) yield
Abid Ilyas Dar1, Fahad Saleem1, Mumtaz Ahmad1, Muhammad Tariq2, Anwar Khan2, Arfan Ali2 , Bushra Tabassum2, Qurban Ali2, Ghazanfar Ali Khan3, Bushra Rashid2, Idrees Ahmad Nasir2,Tayyab Husnain2
Adv. life sci., vol. 2, no. 1, pp. 38-45, November 2014
*- Corresponding Author: Fahad Saleem (fahadawaan@gmail.com)
Author Affiliations
2. Centre of Excellence in Molecular Biology, University of the Punjab Lahore – Pakistan
3. Development and Planning Wing, Department of Agriculture Punjab, Lahore – Pakistan
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Plant growth promoting rhizobacteria (PGPR) play an important role in phosphorous solublization, nutrient uptake and crop productivity. A variety of PGPR and their combinations were supplemented to rice crop for evaluation of their effects on plant height, filled grain per panicle, tillers per plant, 1000 grain weight, panicle length and yield per acre.
Methods: Roots of sugarcane plants and their adhering soil samples were used as an isolation source for PGPR. The nursery plant roots of local rice varieties i.e. Super Basmati and Basmati-515 were inoculated with isolated PGPR formulation. Data was recorded and statistically analyzed to determine analysis of variance, genetic correlation, path coefficient and principle component.
Results: 5 out of 11 bacterial strains produced high indole acetic acid (IAA). Other 6 were either average or low producers of the acid. The strains selected for maximum amount of phosphorous solublization were CEMB-22 (Klebsiella sp.) and CEMB-15 (Burkholderia sp.) with best IAA production. It was found that higher genetic advance, heritability, genotypic and phenotypic correlation have positive direct effects on yield properties of rice.
Conclusion: Yield of rice can be enhanced by the application of CEMB-22+CEMB-15 PGPR in combined formulation.
Key words: Plant growth promoting bacteria, Indole acetic acid, Klebsiella sp., Burkholderia sp, Rice
Introduction
Plant growth promoting bacteria (PGPR) include various groups of bacteria that live freely in the soil and have ability to promote the growth of crop plants through various mechanisms [1-4]. PGPR bacteria play an important role in the establishment of micro-biological niches in the rhizosphere portion of plant in contact with plant roots and soil. They also improve the absorption rate of water, ions, nutrients and soluble salts from soil to plant body; plant vegetative and reproductive potential and hence the yield of different crop [5-8]. The plant roots exude a large number of organic nutrients like organic acids, sugars, vitamins, phytosiderophores, amino acids, nucleosides, mucilage and plant metabolites that attract the micro-organisms through signals of their release in the soil and microbial habitat [9-11]. PGPR bacteria can increase the plant nutrients availability through association of plant and PGPR like phosphate solublization, indole acetic acid (IAA) production and nitrogen fixation [7,12,13]. The root activity of plant may be enhanced through the process of phytosiderophore, enzymatic activities and rhizobial or mycorrhizal symbioses [13,14]. A large number of PGPR can also help crop plants to withstand in abiotic stresses like heavy metals contamination or other pollutants and also enhance the ability of crop plant to sequester the heavy metals. PGPR may also be used as phytoremediation agents to clean soil and environment from heavy metal contamination and other pollutants [15-17]. The present study was conducted to evaluate the effect of PGPR on growth and yield of rice crop (Oryza sativa L.).
Methods
Sampling and isolation of PGPR
The rhizosphere soil samples were collected from sugarcane (Saccharum officinarum L.) plants from different open fields at Lahore, Pakistan in March, 2012. Healthy plants from the sugarcane field were selected and dug out with roots having soil. Roots and adhering soil samples were collected in sterile polythene bags, stored in ice container and brought to CEMB for bacterial isolation, characterization and preservation. 10 grams of each sample along with roots were crushed in 10 mL autoclaved saline solution (0.85% NaCl). 10−1 to 10−6 serial dilution were prepared in normal saline to check the phosphorous solubilize characteristics of the PGPR. 100 uL of each dilution was streaked on National Botanical Research Institute’s Phosphate (NBRIP) media (Nautiyal, 1999) agar plates. Plates were incubated at 28°C for 48 to 72 hours. Halo zone formation due to solubilization of phosphate were observed on the cultured plates. PSB colonies were purified on NBRIP media. 11 strains isolated and glycerol stocks were prepared.
Qualitative analysis of IAA production
All PSB positive colonies were screened for the production of IAA [18]. Isolated colonies were inoculated and incubated at 30±2oC for five days in tryptophan-less L.B media. Centrifugation of all cultures was performed at 13000 rpm for 10 minutes followed by mixing of 2 mL supernatant with two drops of ortho-phosphoric acid and 4 mL of Salkowski’s reagent (50 mL, 35% HLO4; 1.0 mL 0.5 M FeCl3).
Quantitative analysis of soluble P available
Bacterial strains which showed high IAA production activity were selected for quantification of solubilized phosphorous. Bacterial isolates were grown on NBRIP broth containing 1% tricalcium phosphate (TCP). 1 mL of bacterial culture was transferred in broth to incubate at 32°C for 5 days at incubator shaker. After said incubation, cells were separated and supernatant containing soluble phosphorous was quantified by using the vanado-molybedate-yellow color method. Absorbance was measured at wavelength of 430 nm in UV/Visible Spectrophotometer (Beckman Coulter, DU 520). The total soluble phosphorus was calculated from the regression equation of standard curve [19].
Characterization of isolates
DNA was extracted from selected bacterial colonies through CTAB method. Bacterial strains were identified through 16sRNA gene amplification using primers as follow: forward 5’-GGG GTT TGA TCC TGG CTC AG-3’ and reverse 5’- CGG TGT GTA CAA GAC CC -3’ [20]. Annealing temperature of the primers was optimized at 57ºC for 45 seconds. PCR product was checked on 0.8% agarose gel prior to sequencing for confirmation.
Field Trial / Treatments
Field trial was conducted at Research and Development farm of Engro Eximp Agriproducts Pvt. Ltd Muridke, Pakistan, on Super Basmati and Basmati-515 rice varieties during the period of July 2012 to November 2012. Experiment was conducted following randomized complete block design (RCBD) that consisted of four treatments with three replications of both rice varieties. Treatments are shown in table 1 were as follow; T0 for control, T1 treated with CEMB-15, T2 treated with CEMB-22 and T3 treated with mixture of CEMB-15 and CEMB-22. Plot size for each treatment was 500 m2. Soil taken from field for analysis and shown following characteristics: Classified as loam, pH 7.74, Electrical conductivity 1.07m/Scm organic matter 8.4g/kg, available Phosphorous 5.10 mg/kg, and available potassium 75.3mg/kg. Plant height, tillers/plant, panicle length, filled grain/ panicle, thousand grain weight (g), yield kg/acre was recorded from each replication. Plant population and productive tillers / meter2 were recorded by counting the average of three samples (one meter2) taken randomly from each repeat while plant height, grains per panicle, sterility %age, 1000 grain weight were recorded by taking three samples (five plants/sample) taken randomly from each repeat. Data on paddy yield was recorded by harvesting three samples taken randomly from each repeat, each sampling area having size of 25 m2.
Bacterial strains CEMB-15 and CEMB-22 which showed phosphate solubilizing activity (PSA) were applied in 3 replications in the fields. Before rice transplanting, roots dipped in the bacterial culture for 15 to 20 minutes and rest of the culture was flooded in the field. Two rice varieties, Super Basmati and Basmati-515, were selected for field experiments. Both treated and control field were applied with standard fertilizers (2.5 bags NPK 8:23:18) insecticide (cartep 4 g, thiamethoxam) and fungicide (difenoconazole). 33% granular urea and zinc sulphate were supplemented after 15 days of sowing, while potassium, supplemented before panicle initiation was also applied to both treated and control trials with total 20 irrigations to Super Basmati and 19 irrigations to Basmati-515.
Statistical analysis
Data collected were statistically analyzed using Fisher’s analysis of variance techniques and DMRT compared treatment means at 0.05 probabilities (Mstatc). Phenotypic (rp) and genotypic (rg) correlation coefficient was calculated as outlined by Kwon and Torrie [21]. Standard error of genotypic correlation coefficients (SE of rg) were calculated according to Reeve [22] and path coefficient [23] was computed to assess trait association among various grain and its contributing traits [21].
Results
Characterization of PGPR
PSA by selected strains is shown in table 1. Given five days, solubilization of phosphorous reached 40.9% to 62.7% by lowering the pH to 4.0 from initially recorded pH 7. IAA production was categorized on the basis of color intensity. High producers of IAA had high color intensity (figure 2). Among the 11 screened bacterial strains, 5 were high, 3 average and 3 were low IAA producers. Two strains with highest PSA and IAA production (CEMB-22 and CEMB-15) were identified as Klebsiella sp. and Burkholderia sp., respectively.
Nucleotide sequence accession numbers
The 16S rRNA genes of the two bacterial strains (CEMB-22 and CEMB-15) were identified (figure 1) and sequenced and submitted to National Centre for Biotechnology Information web site (www.ncbi.nlm.nhi.gov). The sequence is now public with accession numbers as follow: Burkholderia spp. CEMB-15 (KF487546) and Klebsiella spp. CEMB-22 (KF487553).
Response of PGPR on plant growth and yield traits
Genetic variability
It is evident from table 2 that significant differences were seen for all traits under the application of CEMB-15, CEMB-22, and CEMB-15+CEMB-22 treatment as compared to negative control conditions. Higher heritability was recorded for all studied traits while higher genetic advance was found for tillers per plant and filled grain per panicle. The mean performance of filled grain per plant was 126.170±0.7071 (table 2) while higher filled gain per plant was found for CEMB-15+CEMB-22 (131.00) (table 3) as compared with CEMB-15 (129.00) and CEMB-22 (128.00).
CEMB-15+CEMB-22 was found to be the cause of improved filled grain per panicle. Average plant height was found to be 103.920±1.3878 cm (table 2) while higher filled gain per plant was found for CEMB-15+CEMB-22 (106.67 cm) (table 3) as compared with CEMB-22 (104.33 cm) and CEMB-15 (104.00 cm). Average panicle length 28.183±0.3034 cm (table 3) while CEMB-15+CEMB-22 (29.367 cm) showed higher panicle length as compared with separate applications. CEMB-15+CEMB-22 supplementation (23.667 g) showed higher 1000-grain weight than others (table 3). Average performance for tillers per plant was recorded as 20.000±1.0453 while it was higher for CEMB-15+CEMB-22 (23.667) (table 3) as compared with separate application of CEMB-15 and CEMB-22. It was average grain yield per plant 2085.700±10.262 kg/acre (table 2) and higher for CEMB-15+CEMB-22 (2181.7 kg/acre) as compared with separate application of CEMB-15 and CEMB-22 (table 3).
Genotypic and phenotypic correlation
Genotypic correlation suggested that the traits were controlled directly through genotypic composition of the trait. Significant and strong correlation was found between tillers per plant, panicle length, 1000-grain weight and grain yield per plant (table 4). It is revealed in table 5 that highly significant and strong phenotypic correlation exist for all studied traits.
Positive and significant environmental correlation was found between plant height, panicle length and tillers per plant while negative correlation was found between tillers per plant and panicle length, plant height and 1000-grain weight, filled grain per panicle and yield per plant; and plant height and tillers per plant (table 6).
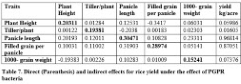
Path coefficient analysis
Direct and indirect effects of yield traits may be used for the selection of higher yielding different genotypes of crops. It is evident from table 7 that plant height showed positive direct effect on yield per plant while positive indirect effects were found for tillers per plant, panicle length and 1000-grain weight while filled grain per plant showed negative indirect effects via plant height on yield per plant. Tillers per plant showed positive direct and indirect effects for plant height, filled grain per panicle and 1000-grain weight while negative indirect effect was observed for panicle length on yield per plant. Panicle length and filled grain per plant showed positive direct effects on yield per plant and also positive indirect effect on yield per plant were found via all studied traits. It was found that 1000-grain weight had positive direct effects on yield per plant and positive indirect effects via tillers per plant, panicle length and filled grain per panicle while negative indirect effects via plant height on yield per plant.
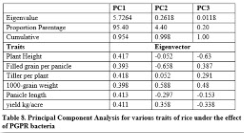
Principal component analysis
It is shown in table 8 that 95.40% variation was present in PC1 while 4.40% and 0.20% for PC2 and PC3 respectively. It was found that higher contributing traits were plant height, tillers per plant, panicle length and yield per plant in PC1. Higher contribution was shown by 1000-grain weight in PC2 and PC3. Negative contribution was found for panicle length, plant height and filled grain per panicle in PC2 and PC3.
Discussion
Different bacteria were screened for phosphate solubilizing bacteria capable of producing halo zones on inorganic source of phosphorous i.e. tricalcium phosphate. These bacteria were screened on NBRIP media in the laboratory as mentioned. In this study, PGPR were found readily solubilizing the insoluble tricalcium phosphate as in already reported cases during research on other crops [24,25].
Different PGPR strains produce IAA whose positive effect on crop is well studied [25]. Previously, it was suggested that secretion of IAA along with phosphate solublization is good tool for Bio Fertilizer technology and have good impact on field [26]. In present stuy, 9 out of 11PSB isolates produced IAA through tryptophan independent pathway. The IAA was maximum after 48 hours of growth and it decreased after 72 hours. According to Chaiharn and Lumyong the best IAA strain Klebsiella pneumonia secreted maximum IAA on 9th day while we observed that our isolate of Klebsiella pneumonia decreased IAA after 48 hours [26]. According to Mohite [27], the IAA production is negligible in L-tryptophan free medium while Ahmed et al., [18] showed that IAA can be secreted by bacteria, in accordance with our study. Findings of present study signifies that the better screening of local isolates of PGPR can be used beneficially. Application of combined CEMB-15+CEMB-22 showed higher effects on improving yield and production of rice. PGPR also played an important role in stress resistant in various crop plants and photosynthetic process. In light of this study, effects of CEMB-15+CEMB-22 on other crops should also be evaluated in order to improve agriculture outputs organic fertilizers through the use of micro-organisms as compared with chemical fertilizers [28,29].
It is suggested from correlation studies that selection on the basis of significant genotypic correlation of traits may be used for developing higher yield rice genotypes [30,31]. Phenotypic and environmental correlations indicated that the application of CEMB-15, CEMB-22 and CEMB-15+CEMB-22 PGPR bacteria might be helpful to improve crop plant yield and productivity of rice. Positive direct and indirect effects also suggested that selection for higher yielding rice genotypes on the basis of direct genetically associated traits may be useful. Similar results were reported for genetic association of various traits by Khalid [6] and Mishra et al. [8]. The positive contribution shown in PC1 suggested that selection on the basis of traits had higher contribution. The accumulation of organic compounds might be enhanced to increase crop yield and productivity. So, it is concluded that the yield of rice could be enhanced by combine application of CEMB-22+CEMB-15 PGPR.
It is also suggested from prescribed study that research should be conducted to find out the effects of CEMB-15+CEMB-22 on other major crops like maize, wheat, sugarcane and cotton. On the other hand, there should be a search for any disadvantageous effects of this supplementation on naturally occurring microbiome.
References
- Wang C, Knill E, Glick BR, Défago G. Effect of transferring 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase genes into Pseudomonas fluorescens strain CHA0 and its gac A derivative CHA96 on their growth-promoting and disease-suppressive capacities. Canadian Journal of Microbiology, (2000); 46(10): 898-907.
- Holguin G, Glick B. Transformation of Azospirillum brasilense Cd with an ACC deaminase gene from Enterobacter cloacae UW4 fused to the Tet r gene promoter improves its fitness and plant growth promoting ability. Microbial ecology, (2003); 46(1): 122-133.
- Hontzeas N, Saleh SS, Glick BR. Changes in gene expression in canola roots induced by ACC-deaminase-containing plant-growth-promoting bacteria. Molecular plant-microbe interactions, (2004); 17(8): 865-871.
- Robison MM, Shah S, Tamot B, Pauls KP, Moffatt BA, et al. Reduced symptoms of Verticillium wilt in transgenic tomato expressing a bacterial ACC deaminase. Molecular Plant Pathology, (2001); 2(3): 135-145.
- Berg G, Smalla K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS microbiology ecology, (2009); 68(1): 1-13.
- Khalid A, Arshad M, Zahir Z. Screening plant growth‐promoting rhizobacteria for improving growth and yield of wheat. Journal of Applied Microbiology, (2004); 96(3): 473-480.
- Mayak S, Tirosh T, Glick BR. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiology and Biochemistry, (2004); 42(6): 565-572.
- Mishra M, Kumar U, Mishra PK, Prakash V. Efficiency of plant growth promoting rhizobacteria for the enhancement of Cicer arietinum L. growth and germination under salinity. Adv Biol Res, (2010); 4(2): 92-96.
- Badri DV, Weir TL, Van Der Lelie D, Vivanco JM. Rhizosphere chemical dialogues: plant–microbe interactions. Current opinion in biotechnology, (2009); 20(6): 642-650.
- Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol, (2006); 57233-266.
- Drogue B, Combes-Meynet E, Moënne-Loccoz Y, Wisniewski-Dyé F, Prigent-Combaret C. Control of the cooperation between plant growth-promoting rhizobacteria and crops by rhizosphere signals. Molecular Microbial Ecology of the Rhizosphere, Two Volume Set, (2013); 281.
- Richardson AE, Barea J-M, McNeill AM, Prigent-Combaret C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant and soil, (2009); 321(1-2): 305-339.
- Lugtenberg B, Kamilova F. Plant-growth-promoting rhizobacteria. Annual review of microbiology, (2009); 63541-556.
- Couillerot O, Prigent‐Combaret C, Caballero‐Mellado J, Moënne‐Loccoz Y. Pseudomonas fluorescens and closely‐related fluorescent pseudomonads as biocontrol agents of soil‐borne phytopathogens. Letters in applied microbiology, (2009); 48(5): 505-512.
- Richardson AE, Simpson RJ. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant physiology, (2011); 156(3): 989-996.
- He Z-l, Yang X-e. Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils. Journal of Zhejiang University Science B, (2007); 8(3): 192-207.
- Tak HI, Ahmad F, Babalola OO (2013) Advances in the application of plant growth-promoting rhizobacteria in phytoremediation of heavy metals. Reviews of Environmental Contamination and Toxicology Volume 223: Springer. pp. 33-52.
- Ahmad F, Ahmad I, Khan MS. Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turk J Biol, (2005); 2929-34.
- Ahmad F, Ahmad I, Khan M. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiological research, (2008); 163(2): 173-181.
- Felske A, Rheims H, Wolterink A, Stackebrandt E, Akkermans AD. Ribosome analysis reveals prominent activity of an uncultured member of the class Actinobacteria in grassland soils. Microbiology, (1997); 143(9): 2983-2989.
- Kwon S, Torrie J. Heritability and interrelationship among traits of two soybean populations. Crop Sci, (1964); 4(2): 196-198.
- Reeve E. The variance of the genetic correlation coefficient. Biometrics, (1955); 11(3): 357-374.
- Dewey DR, Lu K. A correlation and path-coefficient analysis of components of crested wheatgrass seed production. Agronomy Journal, (1959); 51(9): 515-518.
- Yu X, Liu X, Zhu TH, Liu GH, Mao C. Isolation and characterization of phosphate-solubilizing bacteria from walnut and their effect on growth and phosphorus mobilization. Biology and Fertility of Soils, (2011); 47(4): 437-446.
- Azospirillum R. Root-associated Enterobacter and Klebsiella in Poa pratensis: characterization of an iron-scavenging system and a substance stimulating root hair production. Molecular Plant-Microbe Interactions, (1990); 3(6): 358-365.
- Chaiharn M, Lumyong S. Screening and optimization of indole-3-acetic acid production and phosphate solubilization from rhizobacteria aimed at improving plant growth. Current microbiology, (2011); 62(1): 173-181.
- Mohite B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. Journal of soil science and plant nutrition, (2013); 13(3): 638-649.
- Vacheron J, Desbrosses G, Bouffaud M-L, Touraine B, Moënne-Loccoz Y, et al. Plant growth-promoting rhizobacteria and root system functioning. Frontiers in plant science, (2013); 4.
- Ali Q, Ahsan M, Khan NH, Waseem M, Ali F. An overview of Zea mays for the improvement of yield and quality traits through conventional breeding. Nature & Science, (2014); 12(8).
- Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS microbiology Letters, (1999); 170(1): 265-270.
- Ali Q, Ahsan M, Tahir MHN, Basra SMA. Genetic evaluation of maize (Zea mays L.) accessions for growth related seedling traits. International Journal for Agro Veterinary and Medical Sciences, (2012); 6(3): 164-172.