![]()
Phylogenetic analysis of coat protein gene of CYDV-RPV strain
from Wheat
Madiha Zamurrad1, Shahid Hameed2, Irfan Ul Haque1, Kamran Saleem3*, Samra Kausar4
Adv. life sci., vol. 2, no. 1, pp. 16-22, November 2014
*- Corresponding Author: Kamran Saleem (Email: Kammmran@hotmail.com)
Author Affiliations
2-CDRI, National Agriculture Research Center, Islamabad. Pakistan
3-Nuclear Institute for Agriculture and Biology, Faisalabad. Pakistan
4-Adaptive Research Farm, Chakwal, Pakistan
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: Keeping in view the potential damage caused by viruses to production of different crops and possible ‘directed damages’ by manipulated viral attack in/across border collectively make phylogenetic analysis of any attacking viral specie important. Cereal yellow dwarf viruses (CYDV) are highly important viruses in wheat causing significant yield loss.
Methods: Double antibody sandwich ELISA and reverse transcription polymerase chain reaction (RT-PCR) was used to detect and confirm the polerovirus i.e. CYDV-rhopalosiphum padi virus (RPV), and unassigned viruses (SGV, RMV) in Punjab and NWFP provinces. The PCR products were inserted into a pGEM®-T easy vector, which then transformed in JM-107 cells of Escherichia coli. Recombinant plasmids were sequenced. Nucleotide and predicted amino acid sequences were aligned, analyzed and compared with other RPV isolates of the family. The nucleotide sequence data were used to make a phylogenetic tree.
Results: Sequencing of 600 bp of coat protein gene confirmed the presence of CYDV-RPV strain. Pakistani isolate has close phylogenetic relationship with RPV-Mexcio and RPV-Yolo (USA). They had 99.95% similarity with RPV-Pakistan. The RPV-Aus, RPV-IR, and RPV-Cal (USA) had 99.94% identities with RPV-Pakistan.
Conclusion: This work led to a conclusion that there is very low genetic diversity in RPV-Pakistan. Now it is in our future interest to clarify the identity of RPV-PK with more sequencing. The current study may help scientists to formulate appropriate management strategies against CYDV-RPV.
Key words: Phylogeny, Cereal yellow dwarf viruses, Polerovirus, Rhopalosiphum padi virus, Coat protein
Introduction
The diseases of wheat have historically been one of the major biotic production constraints both in Pakistan and the rest of the world. Diseases, especially rusts and powdery mildew are major biotic stresses of wheat crop that inflict heavy losses when in epidemic form. Among the viral diseases, Barley yellow dwarf virus (BYDV) and Cereal yellow dwarf virus (CYDV) cause substantial losses throughout the world e.g. in wheat 17%, barley 15% and oat 25% [1]. BYDV was first reported by Oswald and Houston in 1953 [2] and was confirmed by Watson and Mulligan during 1957 [3] in the United Kingdom. In Pakistan, BYDV has been detected by Khalid in 1992 [4]. The family of BYDV/CYDV is Luteoviridae which has been divided into three genera, i.e. luteovirus, polerovirus and enamovirus, based on differences in the RNA dependent RNA polymerase (RdRp) sequence and structural proteins [5]. BYDV could broadly refer to species of luteovirus (BYDV-MAV and BYDV-PAV), polerovirus (CYDV-RPV) and three unassigned species (BYDV-RMV, BYDV-SGV, and BYDV-GPV) [1]. CYDV-RPV transmitted by vector Rhopalosiphum padi in persistent manner [6]. In many parts of the world Rhopalosiphum padi is considered as the primary risk factor for BYDV/CYDV incidence and aphid related yield loss on cereals [7].
Luterovirus and polerovirus genome is a linear ssRNA of 6 kb that has five to six major open reading frames (ORFs). For most plant viruses, serological tests using virus-specific antibodies and sequence information of ORF3 encoding the coat protein (CP) gene are the two most important criteria in discriminating among species of family Luteoviridae while ORF1/2 fusion protein including polymerase gene are also used, particularly, for distinguishing the genera [8]. Group- I luteoviruses (MAV, PAV) nucleotide sequences contain very small ORFs near the 3’ end of their RNAs that are not present in the nucleotide sequence of the group II poleroviruses (CYDV-RPV, BWYV, PLRV). Similarly, the nucleotide sequences of the group II luteoviruses contain much larger ORFs near their 5’ end than sequences found in the PAV and MAV [6]. The genomic RNA of CYDV-RPV has a small protein (VPg) covalently attached to its 5’end but does not have a poly (A) tail [9,10]. In CYDV/BYDV infected cell, 3’- co-terminal three sgRNAs are produced which play different roles in virus replication [11,12]. sgRNA2 codes for a small 4.3- to 7.0 kDa peptide that is dispensable for virus replication in protoplasts [18]. The role of sgRNA3 which lacks ORF is unclear [13,14]. Phylogenetic analysis reveals that the RNA dependent RNA polymerase (RdRp) genes of subgroup1 and subgroup 11 luteoviruses are different. No other virus group in any kingdom has such an extreme dichotomy in polymerase gene origins. In, contrast coat protein gene of all leutoviruses is more closely related than to the coat protein genes of viruses in any other group [15]. CYDV becoming prevalent and important serotype in all wheat growing areas of the Pakistan. Keeping in view the current dilemma of CYDV the present study was executed for sequencing of coat protein gene and phylogenetic analysis of CYDV-RPV on the basis of CP gene sequence.
Methods
Sampling
Samples on the basis of visual observation of BYDV/CYDV symptoms were collected from wheat field area of National Agriculture Research Center (NARC) Islamabad. Two/three discolored leaves were detached from each affected plant, placed into plastic bags, and stored at 4oC for processing. Healthy plant samples were also taken as negative control from the field and glass house.
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Total plant RNAs from DAS-ELISA CYDV positive plants was extracted using Rneasy Plant Mini Kit of Qiagen kit (catalogue # 74904) according to the manufacturer’s protocol. 0.1 g tissue was ground in liquid nitrogen, mixed 450 µL RLT buffer. Virus specific primers were designed for the coat protein gene for RPV isolate of CYDV according to available sequences of USA isolate of RPV (Accession no. EF521848) obtained from the gene bank of The National Centre for Biotechnological Information (NCBI). Forward Primer BRCF3 (5’-ATGAGTACGGTCGTCCT-3’) and reverse primer BRCR2 (5’-CTATTTTGGGTTTTGTAGC-3’) were designed in Primer3 online tool. Primers sequences were used for reverse transcription and amplification of coat protein gene of CYDV-RPV. cDNA synthesis was achieved using RevertAidtm fermentas Kit (K1691) with 5 µL nucleic acid extract, 1 µL of reverse primer BRCR2 and 6 µL DEPC-treated water in 12 µL reaction. Mixture was incubated at 70oC for 5 min followed by chilling the tubes on ice for 5 min. 5X reaction buffer (4 µL), Ribolocktm RNAse inhibitor (1 µL), 10 mM dNTP (1 µL) and M-MuLV reverse transcriptase (1 µL) were added into reaction mixture. Total volume of the reaction mixture was raised to 20 µL. Reaction was then incubated at 37ºC for 5min, followed by 60 minute treatment at 42ºC. Finally reaction was terminated by heating at 70 ºC for 5 min. for amplification of CP gene through PCR, 50 µL reaction was prepared containing 5 µL of 10x Taq reaction buffer (10mM of Tris-HCL, pH 8.8, 50 mM KCL and 1% (v/v) Nonidet P40), 200 µM dNTP each, 10 pmol of each primers (BRCF3 and BRCR2), 1.5 mM MgCl2, 1U Taq polymerase (Promega M8291) and 2 µL cDNA template. Reaction cycle comprised of an initial step of denaturation for 3 min minutes at 94ºC followed by 35 cycles each consisting of a denaturation step of 30 seconds at 94ºC, an annealing step 30 sec at 51ºC and an extension step of 1min at 72ºC. Seven minutes was given after the last cycle to the extension step at 72ºC to ensure the completion of reaction. Amplified products were analyzed in 1% agarose gel containing 4 µL of ethidium bromide (10 mg/mL) in 1X tris-borate EDTA (TBE) buffer (tris 108 g/l, borate 55g/L, and EDTA 7.5 g/L).
Cloning of CP gene
The amplified product was first ligated into pGEM®-T easy plasmid (Promega, A1360. USA). The ligation was done by adding 1 µL vector, 4 µL PCR product, T4 DNA ligase and 5 µL of 2X Rapid Ligation buffer (40 mM tris-HCL, 10 mM MgCl2, 10 mM DTT, 0.5 mM ATP, pH 7.8). Total ligation mixture (10 µL) was incubated at 4°C overnight and then used for electroporation into electro-competent cells. E. coli JM-107 cells were used for cloning purpose in this study. The cells were prepared according to Sambrook (1989) method. Briefly, a single colony of E. coli JM-107 was cultured overnight in 3 mL Luria-Bertani (LB) medium (tryptone 10 g/L, yeast extract 5 g/L and NaCl 10 g/L, pH 7.0) in a test tube at 37oC. The culture was shifted into 100 mL LB medium in a 250 mL flask at 37oC, until the O.D at 600 nm became 0.4-0.7. Then the cells were transferred to sterile, disposable, ice-cold 50 mL polypropylene tubes (Falcon 2070). Temperature of the cultures were brought down to 00C by storing the tubes on ice for 10 minutes. The cells were pelleted down by centrifugation at 4000 rpm for 10 minutes using Sorvall GS3 rotor (or its equivalent). The pellet was re-suspended in 10 mL of ice-cold 0.1M CaCl2 and store on ice. The cells were again pelleted down by centrifugation at 4000 rpm for 10 minutes using Sorvall GS3 rotor (or its equivalent). The pellet was again re-suspended in 20 mL of ice-cold 0.1 M CaCl2 for each 50 mL of original culture. Dispensed the cells into aliquots in eppendorf tubes and were stored at -70oC. All the centrifugation steps were performed at 40C. The competent cells were mixed with ligation mixture. Tubes were stored on ice for 30 minutes. Then tubes were placed in a circulating water bath that had been preheated to 42oC. The tubes were left in the rack for exactly 30oC and were not shaken at this stage. The tubes were then transferred on ice for 1-2 minutes. 800 µL were added to each tube and incubated at 37oC for one hour on shaking incubator. After incubation, 100 µL of transformed culture was spread on solid LB medium and selected using 50 µg/mL ampicillin, screened for functional β-glucoronidase expression, using 40 µL of 0.1M IPTG (isopropyl- β- D- thiogalactopyranoside) solution per plate with 40 µL of 20 mg/mL X-Gal (5- bromo-4-chloro-3-indolyl- β-D-galactopyranoside) solution per plate. The white colonies (indication of insertional inactivation of lacZ gene by cloned fragment) were picked with sterile tips and cultured in liquid LB medium containing 50 µg/mL ampicillin. The culture tubes were kept at 37oC in orbital shaker incubated overnight with vigorous shaking for isolation of the plasmids. Plasmid from the selected clones was isolated using QIAprep Spin miniprep as per manufacture’s protocol.
Phylogenetic analysis through neighbor joining method
CYDV-RPV coat protein gene Sequences from nine isolates were obtained from NCBI database, verified by a BLAST research of GenBank nucleotide database and used for comparison. Nucleotide sequence of Pakistani isolate RPV-PK was aligned, analyzed and compared with nine isolates of family Luteoviridae available in GenBank using ClustalW2 in MEGA 4.1 [16].
Results
CYDV-RPV specific primers BRCF3 and BRCR2 amplified fragments encompassing the entire CP gene of CYDV-RPV. The molecular product of the expected size of approximately 0.6 kb was obtained (Figure 1). The PCR product was ligated in pGEM®-T easy plasmid vector followed by its transformation into E. coli- JM 107.
Sequencing of CP gene of CYDV-RPV-PK
Although, most of the reported isolates gave PCR product of approximately similar size (0.6 kb). The sequencing of our isolate revealed that the fragment comprise of 615 bp. The CP gene sequence of CYDV-RPV is submitted to NCBI GenBank and accession number HM488009. The sequences were aligned using the program ClustalW2 in MEGA 4.1.
Phlogenetic analysis of CP gene of CYDV-RPV
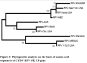
The nucleotide sequence data were used for phylogenetic analysis using MEGA 4.1. The analysis also included the corresponding fragments from the sequences reported as shown in (Table 1). The phylogenetic analysis revealed that the 10 sequences had no clustering on regional basis (Figure 2).
All the CP sequences clustered as one. The Pakistani CP sequence made a sub-cluster with RPV-Mexico and RPV-Yolo (USA). They had 99.95% similarity with RPV-Pakistan. The RPV-Aus, RPV-IR, and RPV-Cal (USA) had 99.94% identities with RPV-Pakistan (Table 2). The GPV CP sequence derived from NCBI database was used as out group in this analysis, maintained its identity as separate virus (Figure 2). The phylogenetic analysis carried out from the nucleotide sequence of CP gene of CYDV-Pakistan clearly indicates that all the isolates from different regions belongs to one group.
The phylogenetic analysis carried out from the nucleotide sequence of CP gene of CYDV-Pakistan clearly indicates that all the isolates from different regions belongs to one group. Although minor cluster developed but there was no geographical separation. As it is very much evident from the phylogram that CYDV-RPV sequence from USA falls into every minor cluster, whereas the CYDV-RPV-Aus maintained a separate lineage. Predicted amino acid sequence of RPV-Pakistan were also aligned, analyzed and compared with other RPV isolates of the family Luteoviridae (Figure 3).
The phylogenetic analysis revealed the same result as obtained on the basis of nucleotide alignment. Similarity between CYDV-RPV-PK and other translated sequences of CYDV-RPV range from 99.89% to 99.913%. Slightly different behavior with 99.89% similarities was maintained by Palmer (Table 3).
Tables & Figures
Figure 1 Figure 2 Figure 3 Table 1 Table 2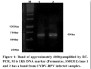



Discussion
Barley yellow dwarf symptoms were first observed in 1951 in California [2] and shortly thereafter were found elsewhere in North America, Europe and Australasia. Now, they are currently active within much of the world’s cereal-growing regions and grasslands, where they suppress grain yield. In Pakistan, BYDV has been detected in 1992 [4]. However, there is no work done on its molecular characterization. This work, which bridges this gap, reveals that the nucleotide sequence of CP of CYDV-RPV-PK has very high sequence identity with other RPV isolates of Luteoviridae family. This is because coat protein of luteoviruses is the most conserved viral gene [17]. CPs are defined by their structural role in encapsidation, which protects the genetic information from degradation [18]. Besides its obvious function in forming virions, the coat protein (encoded by ORF 3) may have roles in virus movement and replication in plants [18]. BYDV also influence grassland dynamics in numerous ways that ecologists are just beginning to understand [19]. Environmental shifts, human activities, introduction of new host species and inadvertent long-distance transport of aphids provide multiple opportunities to alter BYDV epidemiology and cause potential long-term changes in plant and virus communities. The finding of this study, i.e. higher homology of nucleotide sequence of CP from CYDV-RPV-PK with other RPV isolates of Luteoviridae family, endorse the use of CP gene for tracking the origin of this virus family from different parts of the world. CPs are defined by their structural role in encapsidation, which protects the genetic information from degradation [18]. Besides its obvious function in forming virions, the coat protein (encoded by ORF 3) may have roles in virus movement and replication in plants (Shen and Miller, 2004).This work led to a conclusion that there is very low genetic diversity in RPV-Pakistan. Diversity in luteovirus group BYDV-MAV of Pakistani isolate has already been reported according to which BYDV-MAV has similarity with both MAV-Morocco, MAV-CZ and MAV-Sweden [20]. While this was the first such investigation on polerovirus CYDV-RPV.
In a similar study from Iran, CYDV-RPV has nucleotide similarity of 90-91% CYDV-RPS [21]. Moreover, 97% identity at amino acid level of coat protein was reported between Australian and American isolate of CYDV-RPV [22] which are also in line of current study. Contrary to our study, RPV strain of New York (NY-RPV) was reported to have low coat protein nucleotide and deduced amino sequence similarity (55.2 and 48.8%) respectively [23]. Determination of nucleotide sequence is important to design and prepare rapid molecular detection methods and transgenic plants [24]. So, our study in future may help scientist to develop coat protein mediated resistance, an environmentally safe approach against BYDV/CYDV.
Acknowledgements: The authors gratefully acknowledge the financial assistance of National Agriculture Research Center.
References
- Miller and WA, Rasochová L. Barley yellow dwarf viruses. Annual review of phytopathology, (1997); 35(1): 167-190.
- Guy P, Johnstone G, Morris D. Barley yellow dwarf viruses in, and aphids on, grasses (including cereals) in Tasmania. Crop and Pasture Science, (1987); 38(1): 139-152.
- Watson MA, Mulligan T. Cereal yellow dwarf virus in Great Britain. Plant Pathology, (1957); 6(1): 12-14.
- Siddiqui NN, Ilyas M, Mansoor S, Azhar A, Saeed M. Cloning and phylogenetic analysis of coat protein of barley yellow dwarf virus isolates from different regions of pakistan. Journal of Phytopathology, (2012); 160(1): 13-18.
- Takahashi T, Kojima M, Ohshima K, Uyeda I, Shikata E. Physical and chemical properties of japanese isolate of barley yellow dwarf virus. Journal of the Faculty of Agriculture, Hokkaido University, (1988); 63(4): 363-372.
- Irwin M, Thresh J. Epidemiology of barley yellow dwarf: a study in ecological complexity. Annual review of phytopathology, (1990); 28(1): 393-424.
- Chapin JW, Thomas JS, Gray SM, Smith DM, Halbert SE. Seasonal abundance of aphids (Homoptera: Aphididae) in wheat and their role as barley yellow dwarf virus vectors in the South Carolina coastal plain. Journal of economic entomology, (2001); 94(2): 410-421.
- Ahmad YA, Rassaby L, Royer M, Borg Z, Braithwaite K, et al. Yellow leaf of sugarcane is caused by at least three different genotypes of sugarcane yellow leaf virus, one of which predominates on the Island of Réunion. Archives of virology, (2006); 151(7): 1355-1371.
- Murphy JF, D'Arcy CJ, Clark JM. Barley yellow dwarf virus RNA has a 5′-terminal genome-linked protein. Journal of general virology, (1989); 70(8): 2253-2256.
- Mayo M, Ziegler-Graff V. Molecular biology of luteoviruses. Advances in virus research, (1996); 46(1): 413-460.
- Dinesh-Kumar S, Brault V. Precise mapping and in vitro translation of a trifunctional subgenomic RNA of barley yellow dwarf virus. Virology, (1992); 187(2): 711-722.
- Kelly L, Gerlach WL, Waterhouse PM. Characterisation of the subgenomic RNAs of an Australian isolate of barley yellow dwarf luteovirus. Virology, (1994); 202(2): 565-573.
- Allen Miller W, Hercus T, Waterhouse PM, Gerlach WL. A satellite RNA of barley yellow dwarf virus contains a novel hammerhead structure in the self-cleavage domain. Virology, (1991); 183(2): 711-720.
- Miller WA, Dinesh-Kumar S, Paul CP. Luteovirus gene expression. Critical Reviews in plant sciences, (1995); 14(3): 179-211.
- Gibbs A, Mackenzie A. A primer pair for amplifying part of the genome of all potyvirids by RT-PCR. Journal of Virological Methods, (1997); 63(1): 9-16.
- Kumar S, Nei M, Dudley J, Tamura K. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Briefings in bioinformatics, (2008); 9(4): 299-306.
- Chay C, Gunasinge U, Dinesh-Kumar S, Miller W, GRAY SM. Aphid transmission and systemic plant infection determinants of barley yellow dwarf luteovirus-PAV are contained in the coat protein readthrough domain and 17-kDa protein, respectively. Virology, (1996); 219(1): 57-65.
- Lazarowitz SG, Beachy RN. Viral movement proteins as probes for intracellular and intercellular trafficking in plants. The Plant Cell Online, (1999); 11(4): 535-548.
- Malmstrom CM, Shu R. Multiplexed RT-PCR for streamlined detection and separation of barley and cereal yellow dwarf viruses. Journal of virological methods, (2004); 120(1): 69-78.
- Saleem K, Hameed S, Ul-Haque I. Phylogenetic analysis of coat protein gene of BYDV-MAV strain from wheat. Archives of Phytopathology and Plant Protection, (2013); 46(14): 1747-1755.
- Rastgou M, Khatabi B, Izadpanah K, Kvarnheden A. Nucleotide sequences of a part of Barley yellow dwarf virus-PAV and Cereal yellow dwarf virus-RPV genome from Iran. Parasitica, (2005); 61(1): 89-94.
- Wang M-B, Cheng Z, Keese P, Graham M, Larkin P, et al. Comparison of the coat protein, movement protein and RNA polymerase gene sequences of Australian, Chinese, and American isolates of barley yellow dwarf virus transmitted by Rhopalosiphum padi. Archives of virology, (1998); 143(5): 1005-1013.
- Vincent J, Ueng P, Lister R, Larkins B. Nucleotide sequences of coat protein genes for three isolates of barley yellow dwarf virus and their relationships to other luteovirus coat protein sequences. Journal of general virology, (1990); 71(12): 2791-2799.
- Miller WA, Liu S, Beckett R. Barley yellow dwarf virus: Luteoviridae or Tombusviridae? Molecular Plant Pathology, (2002); 3(4): 177-183.





