![]()
Genetic effects of Calotropis procera CpTIP1 gene on fiber quality in cotton (Gossypium hirsutum)
Sidra Akhtar1, Ahmad Ali Shahid1*, Abdul Qayyum Rao1, Kamran Shehzad Bajwa1, Adnan Muzaffar1, Ayesha Latif1, Tayyab Husnain1
Adv. life sci., vol. 1, no. 4, pp. 223-230, August 2014
*- Corresponding Author: Ahmad Ali Shahid (Email: ahmadali.shahid@gmail.com)
Author Affiliations
Abstract![]()
Introduction
Methods
Results
Discussion
References
Abstract
Background: The importance of cotton crop (Gossypium hirsutum) in textile industry is based on its fiber quality. A number of fiber-specific genes play important role in the development of cotton fiber. Previous studies have demonstrated the importance of genes that are responsible for metabolic functions and their involvement in cotton fiber development.
Methods: This study was focused at successful Agrobacterium mediated stable transformation of the fiber gene CpTIP1, isolated from the wild plant Calotropis procera, into cotton variety NIAB-846 for one generation.
Results: Transformation efficiency was calculated to be 1.01% for the target gene. Different molecular techniques such as PCR were used for confirmation and Real-Time PCR was used to check the level of quantitative expression of fiber expansin gene in putative transgenic cotton plants. On the base of molecular analysis, results showed higher expression level of fiber gene (CpTIP1) in transgenic plants as compared to the control plants.
Conclusion: The results of this study support the idea of improved cotton fiber through genetic modification especially the cotton fiber strength.
Key words: Cotton fiber, CpTIP1 gene, transformation, Agrobacterium
Introduction
Cotton is one of the major crops grown worldwide for fiber and feed. Economically, the most significant product obtained from the cotton plant is the lint which provides high quality fiber to textile industry [1]. Cotton seed is also used for production of cotton oil seed and livestock feed. The other essential products include cotton oil obtained from cotton seeds and cotton seed meal that is used as livestock feed. Though conventional breeding has been playing a substantial role in the improvement of cotton by introducing new alleles but genetic engineering provides a direct method of plant breeding that particularly targets one or a few traits for enhancement of the crop plant.
Changes in growth and development of the cotton fiber form the basis for manipulation of fiber properties for use in the textile industry. There are four distinctive stages that involve in cotton fiber development such as fiber initiation, elongation, secondary cell wall biosynthesis, and maturation. These stages are correlated with each other during the formation of mature fiber [2,3]. Alterations in fiber perimeter affect the length, strength and micronaire of the cotton fibers. The biological mechanisms which control the cell wall expansion and cell diameter regulate the fiber perimeter. Fiber quality can be improved by taking several parameters into account like fiber length, fiber strength, fineness and uniformity. These qualities can be enhanced by introducing genes from bacteria and higher plants [4].
Calotropis procera belongs to family Asclepiadaceae and is commonly known as Giant milkweed. The seeds of the C. procera bear clusters of very fine and strong fibers that are hollow [5]. The length of these fibers depends upon their position within the fruiting body. The seeds have tufts of very fine and strong hollow fibers that range in length from 2.5-4.5 cm depending upon their position within the fruiting body. These fibers are botanically single celled seed trichomes. The C. procera bast fibers have been evaluated for their fiber characteristics in making cloth at a 1:1 ratio with cotton fiber [6]. The yarn has enough potential in natural fiber-reinforced composites and other industrial textile applications [3]. The floss may also substitute cotton wool for surgical or stuffing purposes [7]. It is found in waste lands and grows as a weed in cultivated areas. It also grows well on rubbish heaps, waste and fallow land, by the roadside and in sand dunes. The inner bark of Calotropis is used to make strong fibers called madar which are used in the manufacture of weave carpets, ropes, sewing thread and fishing nets.
Genetic transformation has been used for modification of more than 120 plant species [8]. The preference of Agrobacterium-mediated transformation method over particle bombardment method of gene transfer is based upon the regeneration abilities of local cotton varieties [3].
As available cotton cultivars in the field are not fulfilling the demand of textile industry therefore, it is need of the hour to produce such superior quality fiber varieties that can fulfill the requirements of the textile sector. This study was carried out to transform the fiber gene CpTIP1 from C. procera into cotton cultivar (NIAB-846) via Agrobacterium in order to improve the fiber characteristics.
Methods
Selection of a suitable variety
Fifteen local cotton varieties, specifically, CIM 497, NIAB 846, CIM446, CIM499, CIM473, CIM 443, BH 118, BH 75, BH 79, BH 95, BH 557, MNH 93, NIAB 78, FH 672 and FH 673, were screened for transformation on the basis of their germination percentage. The selected variety of cotton crop for this experiment was NIAB-846. The reason for this selection was that the germination rate of this variety was high as compared to other varieties but the fiber quality was low. Therefore NIAB-846 variety was selected for the transformation of fiber gene CpTIP1 in order to enhance its fiber characteristics.
Agrobacterium mediated transformation
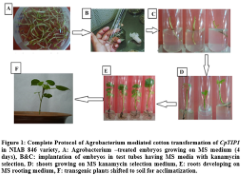
The cotton seeds were delinted by applying commercial sulphuric acid (H2SO4). 0.1% HgCl2 and 0.1% sodium dodecyl sulphate (SDS) were used for sterilization of delinting cotton seeds of NIAB-846 variety. Sterilized seeds were placed in seed germinator at 30 °C overnight under dark condition. In Agrobacterium mediated transformation method, these germinated embryos were used for fiber gene transfer [7,9-11]. For culturing the inoculated explants, MS medium was used. Furthermore 1 mg/L Kinetin and cefatoxime (250mg/L) was used in MS plates for first three days afterwards these plantlets were transferred to MS tubes containing 50 mg/ml kanamycin, 0.5 mg/L benzylaminopurine (BAP) and 1 mg/L α-naphthaleneacetic acid (NAA). After confirmation of the putative transgenic plantlets on selection media, these plants were shifted to soil pots with clay, sand and peat moss (1:1:1) for acclimatization. After acclimatization these putative transgenic cotton plants were shifted to greenhouse for further molecular analysis.
PCR analysis of putative transgenic plants
Genomic DNA was isolated from the putative transgenic plants by using G-Spin™ IIp Genomic DNA Isolation Kit (Catalog No: IB-17271). The concentration and quantity of isolated genomic DNA was then checked with the help of Agarose gel electrophoresis. PCR analysis of the genomic DNA was carried out in order to confirm the presence of transgene in subject plants. For this purpose 4µl of genomic DNA was used as template, 2µl of 10X PCR buffer (Fermentas cat# B34), 2µl of 2mM dNTPs, 1µl of MgCl2 (Fermentas cat# R0971), 2µl of 10 p-mol forward primer (5’ TCTTTGCAGGTCAAGGCTCT 3’), 2µl of 10 pmol reverse primer (5’ ATTTGCTCCCACGATGAAAC 3’) and 0.5 µl of 5u Taq polymerase enzyme (Fermentas cat# EP0071) were used. The annealing temperature for this set of primers was optimized at 62.10C to amplify the 458 bp product size of CpTIP1 gene in putative transgenic cotton plants.
Quantitative expression of transgenes (RNA extraction and qRT-PCR)
The extraction of RNA from newly emerged young leaves of transgenic and a control plant was performed as described earlier [11]. Currently, the standard protocol to measure mRNA that offers the best sensitivity, dynamic range, and reproducibility is quantitative real-time PCR. Using qRT-PCR (Maxima SYBR Green/ROX qPCR Master Mix (2X), Catlog # K0221, Thermo Scientific, USA), mRNA transcripts were reverse transcribed into cDNA using oligo (dt) primers. The cDNA of CpTIP1 was then exponentially amplified with PCR using gene-specific primers. The concentration of the amplicon was monitored with SYBR Green dye. The cDNA of six transgenic plants with the CpTIP1 fiber gene were quantified with qRT-PCR. The qRT-PCR was performed using forward primer (5’-GGTGCATTAGTCGAACCAT-3’) and reverse primer (5’-GACGATCCCAGCTCGATATT-3’). The endogenous expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control. All PCR reactions were performed with the following conditions: 94 ºC for 3 min and 40 cycles of 45 s at 94 ºC, 45 sec at 52 ºC, and 45 s at 72 ºC. Each qRT-PCR sample had three replicates [12].
Results
Agrobacterium mediated cotton transformation of CpTIP1
In order to incorporate the CpTIP1 gene in NIAB 846, Agrobacterium mediated transformation was used. A total of 5000 embryos were transformed with CpTIP1 gene and screening of putative transgenic plants on MS medium with 50 mg/ml kanamycin as a marker. 50 putative transgenic plants with an efficiency of 1.01% out of a total of 5000 transformed embryos were obtained and shifted to MS medium with kanamycin selection. After 1 week of transformation, growing embryos were shifted to selection medium in tubes containing MS medium (50 mg/ml kanamycin). Non-transgenic plants died after 3 days of shifting while transgenic plants survived on the selection medium and showed healthy growth (Figure1).
Detection of CpTIP1 fiber gene in putative transgenic cotton plants
PCR analysis was used for the confirmation
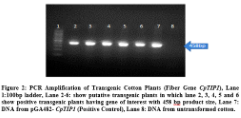
of putative transgenic cotton plants with CpTIP1 fiber gene. For this purpose genomic DNA of seven putative transgenic plants was taken and amplified through PCR by using gene specific primers. Gene specific primers were amplified 458bp product size of gene of interest. Total of 5 plants were detected positive out of 50 putative transgenic cotton plants. No amplification was detected in negative control plant (Figure 2).
Quantification of CpTIP1 fiber gene mRNA in transgenic cotton plants
The quantity of isolated RNA was estimated by using Nano-Drop ND-1000 spectrophotometer. For this purpose 1µl of the deionized water was run as blank that was used for the elution of RNA to avoid any contamination. Then 1µl of RNA sample was loaded to estimate the concentration of RNA in ng/µl. OD of the isolated RNA was estimated by 260/280 and 260/230 values. At the end a report was obtained based on the values of different samples estimated (Table 1).
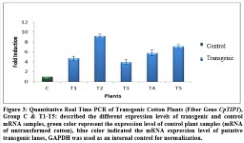
Expression of the transgene, CpTIP1, was checked by Real Time PCR. For this purpose RNA of control and transgenic plants was extracted and cDNA was synthesized. This cDNA was then used as template in RT-PCR. The improvement in quantitative expression of the target gene was varied among in transgenic plants as compared to control plants which are given below. GAPDH gene was used as the reference gene to normalize the expression levels. In T1 (first transgenic plant) expression of the CpTIP1 gene was 2.5 folds higher, in T2 it was 5.2 folds higher than control plant, in T3 it was 1.5 folds higher, in P4 it was 3 folds higher and in T5 produce 3.5 fold more expression as compared to control (Figure 3).
Discussion
Betterment of cotton fiber characteristics for improving strength, length and finesse has been a major goal of many research groups. To achieve these goals efforts are being made through both conventional breeding and genetic engineering tools [7,13]. During the last century of advanced molecular research on model plant such as Arabidopsis produce a correlation between plant cell differentiation and developmental stages of cotton. The hidden aspects of basic procedures of cotton fruit development from initiation to elongation of cellulose rich fiber became clear due to the recent advancements in genome studies. The biochemical pathways of cotton fiber development are controlled by many types of signal transduction and transcriptional regulation components [14].
Transformation of fiber related genes in cotton have a great potential as an agronomic trait. For this purpose many attempts are being made to improve the quality of cotton fiber. Present study highlights the importance of transformation of fiber gene isolated form C. procera in cotton variety NIAB-846 and its molecular analysis. Transformation of fiber genes in cotton is done to improve the quality of the cotton fiber [14]. Similar attempt was made earlier by researchers who transformed acsA and acsB, prokaryotic genes which are involved in cellulose synthesis in Acetobacter xylinum and played an important role in improving cotton quality [12].
In the current study Agrobacterium mediated transformation of CpTIP1 gene was done and 1.01% transformation efficiency was reported which was similar to [15] in which he reported 1.1% transformation efficiency in case of Phytochrome B gene transformation in cotton crop in which he reported 1.24% transformation efficiency in the case of fiber expansin gene transformation in cotton crop [14].
The expression of CpTIP1 was also confirmed initially by Real Time PCR at mRNA level. The putative transgenic plants showed many fold increased CpTIP1 gene expression as compared to the non-transgenic control plants. These results coincide with the reported results in which the increased expression of GhACT1 gene that plays a vital role in fiber elongation and has reported increase expression of CpEXPA3 gene that improve fiber strength of cotton crop [11,14]. Multiple genes are involved in development of fiber quality. It is also reported that production of very strong, fine and long fiber of Calotropis procera is due to the expression of expansin genes during fiber development [16].
The results of present study clearly demonstrated the successful Agrobacterium mediated transformation of the fiber gene, CpTIP1, into the cotton variety NIAB-846 by the Shoot Apex method. The genetic modification of cotton crop through stable cotton transformation for its improved fiber quality has opened new avenues for the progress of the National Textile Industry. Future prospects to perform experiments more rapidly through altering cotton fiber wall properties via fiber genes transformed from different biological sources may contribute towards the improvement of overall cotton fiber quality.
Acknowledgements
We are highly thankful to the Director, Nuclear Institute of Agriculture and Biotechnology (NIAB), Faisalabad, Pakistan for providing seeds of cotton variety NIAB-846 and also grateful to APTMA office, Lahore, Pakistan for allowing us to use fiber analysis equipment’s. We are also thankful to Higher Education Commission (HEC), Pakistan for providing research funding for this project.
References
- Chakravarthy VS, Reddy TP, Reddy VD, Rao KV. Current status of genetic engineering in cotton (Gossypium hirsutum L): an assessment. Critical reviews in biotechnology, (2014); 34(2): 144-160.
- Kim HJ, Triplett BA. Cotton fiber growth in planta and in vitro. Models for plant cell elongation and cell wall biogenesis. Plant physiology, (2001); 127(4): 1361-1366.
- Sakthivel J, Mukhopadhyay S, Palanisamy N. Some studies on Mudar fibers. Journal of industrial textiles, (2005); 35(1): 63-76.
- Saito T, Shibata I, Isogai A, Suguri N, Sumikawa N. Distribution of carboxylate groups introduced into cotton linters by the TEMPO-mediated oxidation. Carbohydrate polymers, (2005); 61(4): 414-419.
- Cheema HMN, Bashir A, Khatoon A, Iqbal N, Zafar Y, et al. Molecular characterization and transcriptome profiling of expansin genes isolated from Calotropis procera fibers. Electronic Journal of Biotechnology, (2010); 13(6): 10-11.
- Varshney A, Bhoi K. Cloth from bast fibre of the Calotropis procera (Aak) plant. Biological wastes, (1988); 26(3): 229-232.
- Hussain SS, Husnain T, Riazuddin S. Sonication assisted Agrobacterium mediated transformation (SAAT): An alternative method for cotton transformation. Pakistan Journal of Botany, (2007); 39(1): 223.
- Griffiths BS, Caul S, Thompson J, Birch ANE, Scrimgeour C, et al. Soil Microbial and Faunal Community Responses to Maize and Insecticide in Two Soils. Journal of environmental quality, (2006); 35(3): 734-741.
- Bakhsh A, Rao AQ, Shahid AA, Husnain T, Riazuddin S. Insect resistance and risk assessment studies in advance lines of Bt cotton harboring Cry1Ac and Cry2A genes. American-Eurasian Journal of Agricultural and Environmental Sciences, (2009); 6(1): 1-11.
- An C, Saha S, Jenkins JN, Scheffler BE, Wilkins TA, et al. Transcriptome profiling, sequence characterization, and SNP-based chromosomal assignment of the EXPANSIN genes in cotton. Molecular Genetics and Genomics, (2007); 278(5): 539-553.
- Rashid B, Saleem Z, Husnain T, Riazuddin S. Transformation and inheritance of Bt genes inGossypium hirsutum. Journal of Plant Biology, (2008); 51(4): 248-254.
- Rao AQ, Irfan M, Saleem Z, Nasir IA, Riazuddin S, et al. Overexpression of the phytochrome B gene from Arabidopsis thaliana increases plant growth and yield of cotton (Gossypium hirsutum). Journal of Zhejiang University SCIENCE B, (2011); 12(4): 326-334.
- Li X, Wang X, Zhao X, Dutt Y. Improvement of cotton fiber quality by transforming the acsA and acsB genes into Gossypium hirsutum L. by means of vacuum infiltration. Plant cell reports, (2004); 22(9): 691-697.
- Bajwa KS, Shahid AA, Rao AQ, Kiani MS, Ashraf MA, et al. Expression of Calotropis procera expansin gene CpEXPA3 enhances cotton fibre strength. Australian Journal of Crop Sciences, (2013); 7(2): 206
- Rao AQ, Bakhsh A, Nasir IA, Riazuddin S, Husnain T. Phytochrome B mRNA expression enhances biomass yield and physiology of cotton plants. African Journal of Biotechnology, (2013); 10(10): 1818-1826.
- Aziz RK, Breitbart M, Edwards RA. Transposases are the most abundant, most ubiquitous genes in nature. Nucleic acids research, (2010); 38(13): 4207-17.